Decoding How Sponsors Evaluate Research Sites for Clinical Trials
When selecting clinical research sites for their trials, sponsors look for a blend of capabilities, resources, and a proven track record that promises the best outcomes for their studies. With sponsors seeking top-performing sites that align with their trial objectives, the ability to showcase expertise, efficiency, and reliability is key component of successful to success. This selection process is crucial as the chosen sites directly impact the efficiency, compliance, and ultimately the success of the clinical trials.
In this blog, we’ll explore the key factors that sponsors consider when evaluating potential clinical research sites. From the expertise of the staff and the facility’s infrastructure to patient recruitment strategies and regulatory compliance, understanding what sponsors are looking for can help clinical sites not only meet but exceed these expectations. Whether you’re a seasoned investigator or a site looking to attract more trials, this insight into sponsor priorities will provide valuable guidance on how clinical research sites can use purpose-built technology, like PROPEL, built by Devana Solutions, to demonstrate that they are a high-performing site that stands out in a competitive field.
Site Experience and Expertise
One of the primary considerations for sponsors during site selection is experience and expertise. Sponsors prefer sites with a proven track record of successfully conducting similar trials, demonstrated by a history of patient recruitment, retention, and data quality. Additionally, sites and Physician Investigators (PIs) having expertise in specific therapeutic areas or specialized procedures relevant to the trial protocol is also highly valued.
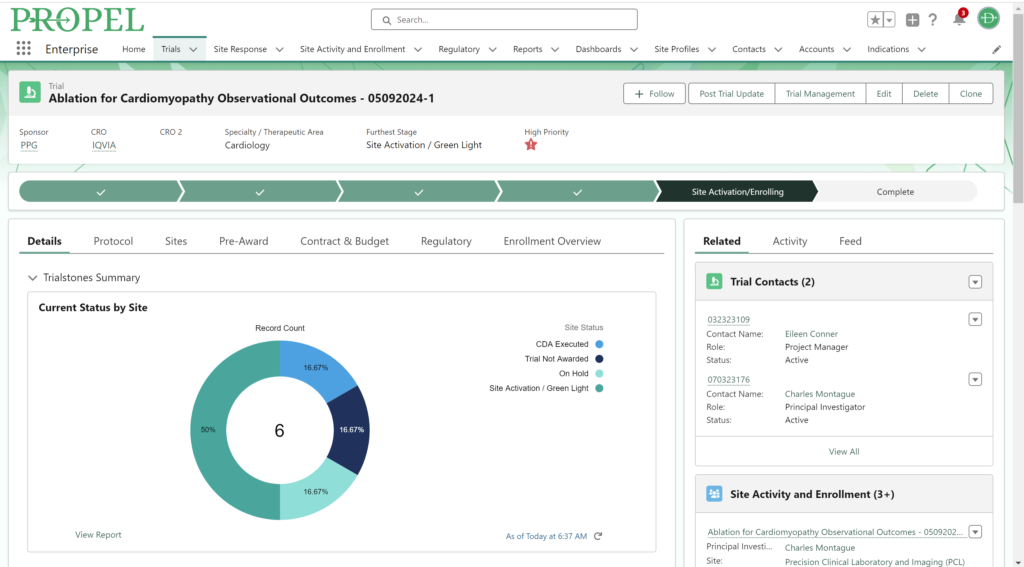
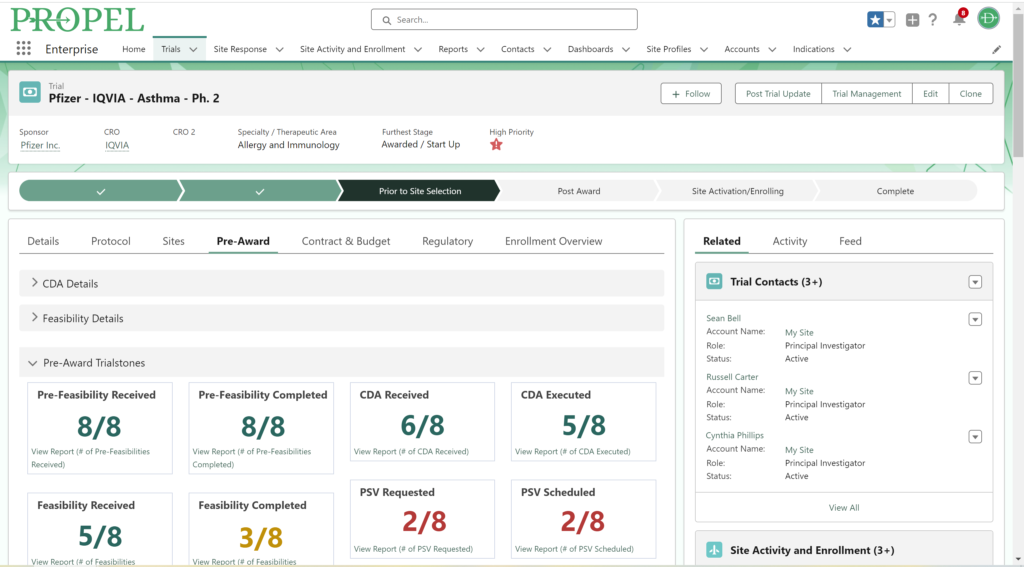
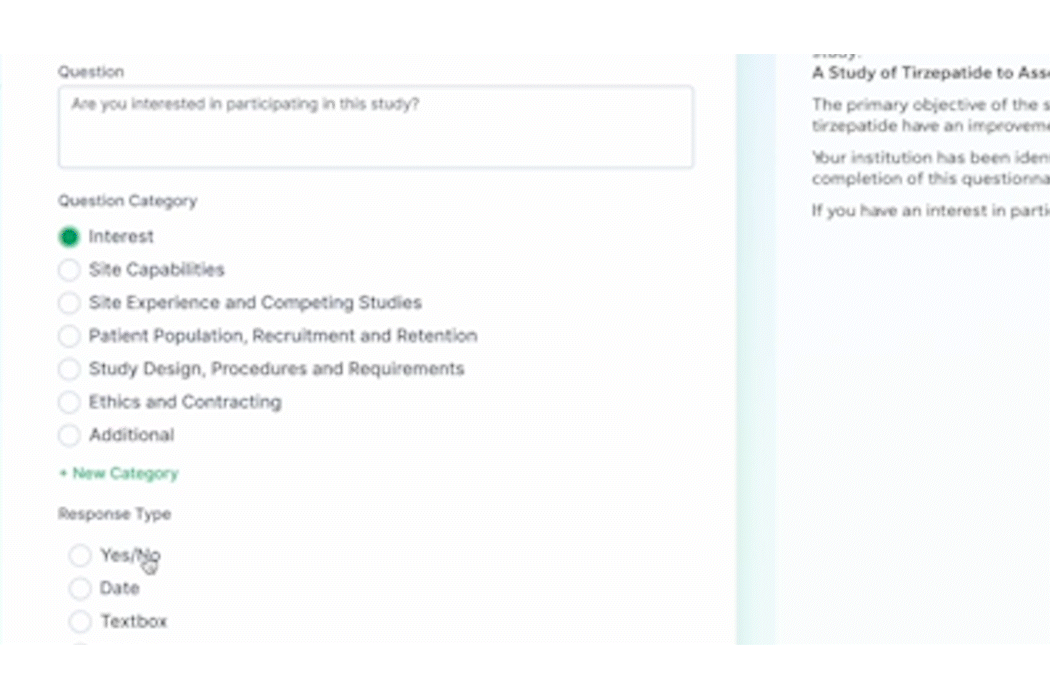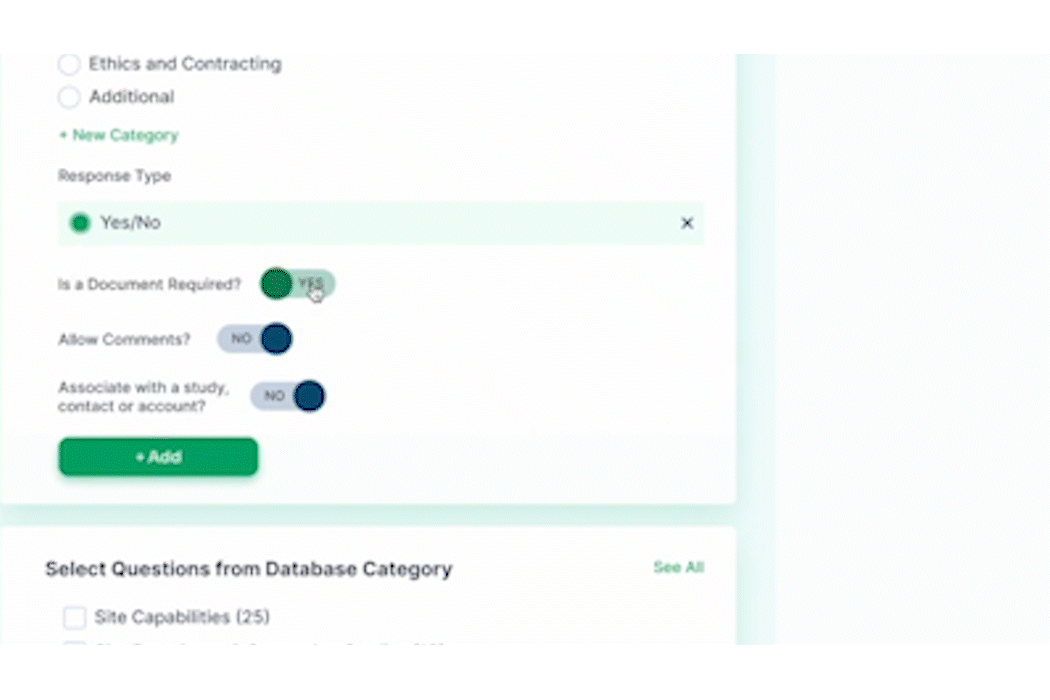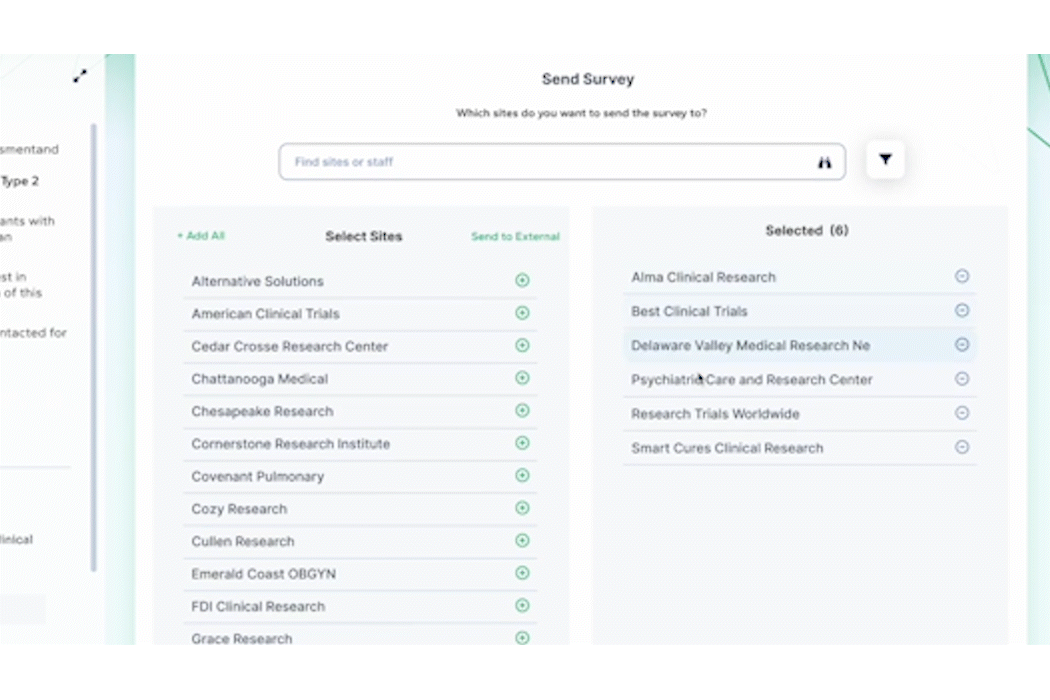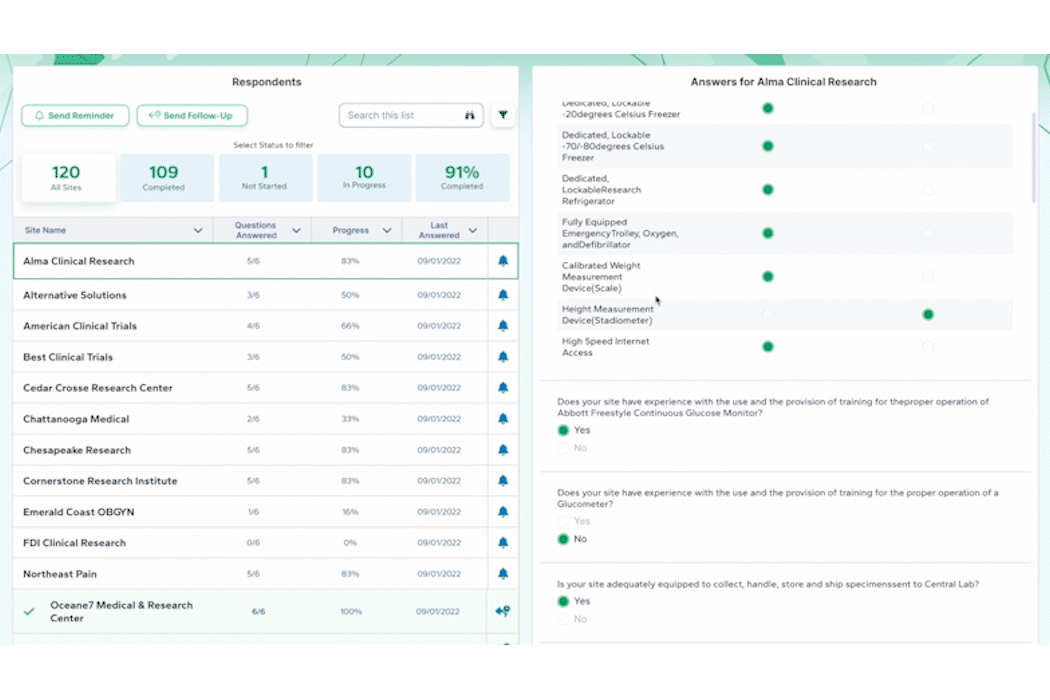
By centralizing data management, PROPEL is a pioneering platform that enables sites to efficiently track and report on key performance metrics. This consolidation of data within a single system streamlines operations, offering unprecedented visibility into trial progress and site performance. With its powerful analytics and reporting capabilities, PROPEL enables research sites to transparently showcase their past and current performance metrics to sponsors. From patient recruitment statistics in a given indication to site activation timelines, PROPEL generates customizable reports highlighting a site’s efficiency and effectiveness. With PROPEL, sites can effectively monitor their performance, identify areas for improvement, and demonstrate their capabilities to sponsors with unparalleled precision and efficiency. Sponsors appreciate sites that can provide transparent and timely performance data, as it allows them to make informed decisions when selecting partners for their trials. Moreover, this transparency enhances the site’s reputation and ultimately strengthens the overall partnership between sites and sponsors.
Patient Population and Recruitment Potential
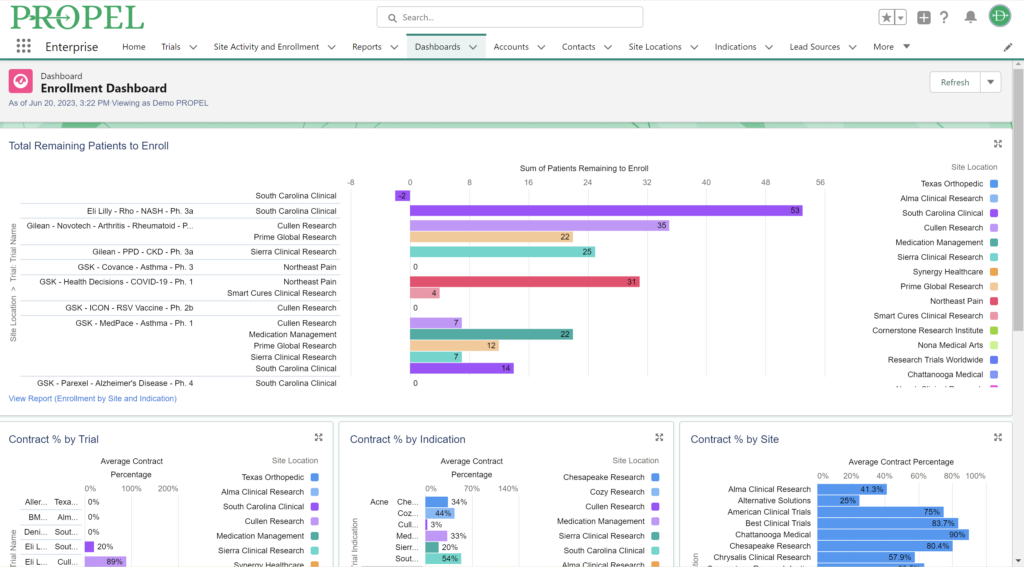
Patient retention and recruitment is another pivotal factor for success in clinical trials. Access to diverse patient pools is particularly important. Clinical research sites are assessed by sponsors based on their access to the target patient population and their innovative strategies for patient recruitment. Sponsors assess clinical research sites based on their access to the target patient population and their strategies for patient recruitment.
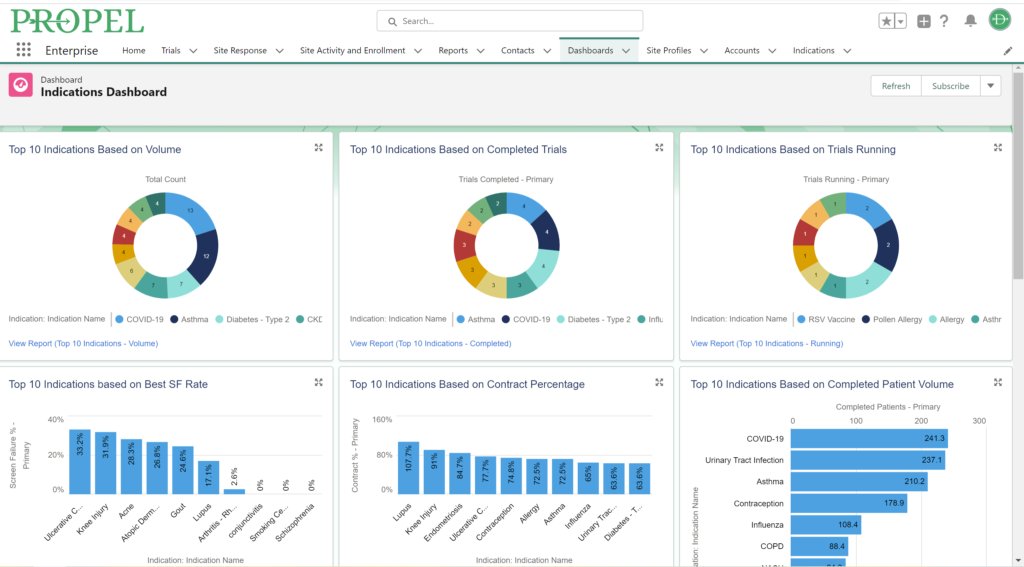
In recent years, there has been a growing mandate to improve diversity in clinical trial populations. This push is driven by the recognition that diverse populations may respond differently to treatments, which has significant implications for the generalizability of trial results. In fact, regulatory bodies like the FDA in the United States have issued guidelines that encourage the inclusion of varied demographic groups in clinical trials to ensure that the findings are applicable to a broader population. These guidelines recommend that trial designs consider factors such as age, gender, and racial and ethnic backgrounds. Sites that have established relationships with patient communities often have an advantage. These relationships facilitate trust and communication, which are crucial for encouraging participation and ensuring participants remain engaged throughout the trial.
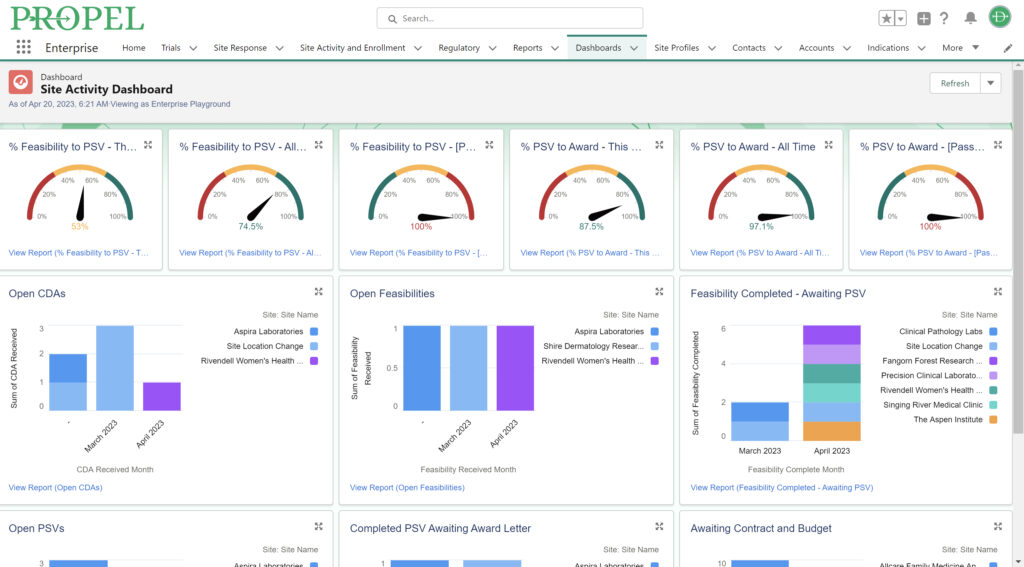
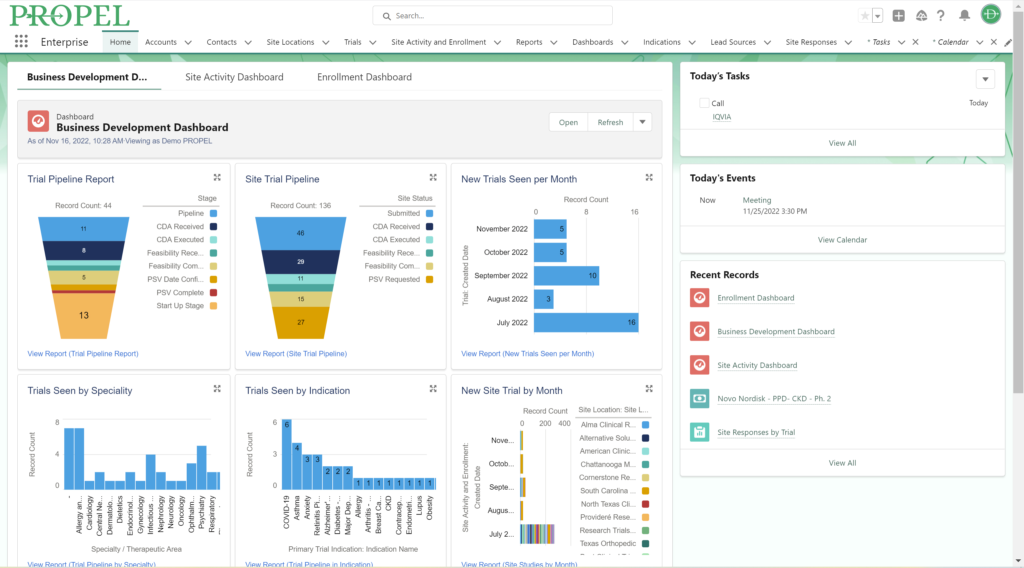
Sponsors value sites that can leverage past and real-time data analytics to drive continuous improvement and ensure the success of their trials. PROPEL’s advanced analytics capabilities provide clinical research sites with invaluable insights into trial performance. By analyzing site metrics, patient recruitment trends, and operational efficiency indicators, sites can make data-driven decisions that optimize trial strategies and resource allocation. Additionally, sites can track which patient recruitment methods have worked the best in the past to inform future decisions. Plus, PROPEL’s seamless integrations with the leading CTMS providers allows for a full-cycle view of past trial performance and patient recruitment.
Site Facility and Resources
The physical infrastructure and resources available at a site are carefully evaluated by sponsors. This includes the availability of state-of-the-art facilities for patient visits, laboratory capabilities for sample processing, and access to necessary equipment and technology. Adequate staffing, including trained research personnel and support staff, is essential for efficient trial conduct.
Using comprehensive site profiles, PROPEL makes it easy to see all the key capabilities, equipment, and staff members in one place. For larger site networks, PROPEL enables central teams to query all their sites and filter by specific criteria, including equipment and therapeutic specialties. This makes it easy for research site organizations of all sizes to respond quickly to sponsors about how their available resources match up to the trial opportunity.
Regulatory Compliance and Quality Assurance
Sponsors are increasingly vigilant about regulatory compliance, given its impact on the integrity and validity of trial results. Sites that can demonstrate that they prioritize and manage regulatory aspects effectively are often more attractive to sponsors. This includes compliance with Good Clinical Practice (GCP) guidelines, adherence to protocol requirements, and timely reporting of adverse events. Sites with robust quality assurance processes and a culture of continuous improvement tend to be favored by sponsors.
PROPEL simplifies regulatory compliance for sites, offering a comprehensive platform for tracking all necessary regulatory documents. The platform includes automated compliance workflows, which significantly reduces the potential for human error and improves the efficiency of compliance processes. Furthermore, PROPEL provides detailed audit trails for sites to consistently verify and document their compliance status throughout the clinical trial process. Altogether, PROPEL helps sites demonstrate their commitment to maintaining high-quality standards.
Budget and Contract Negotiation
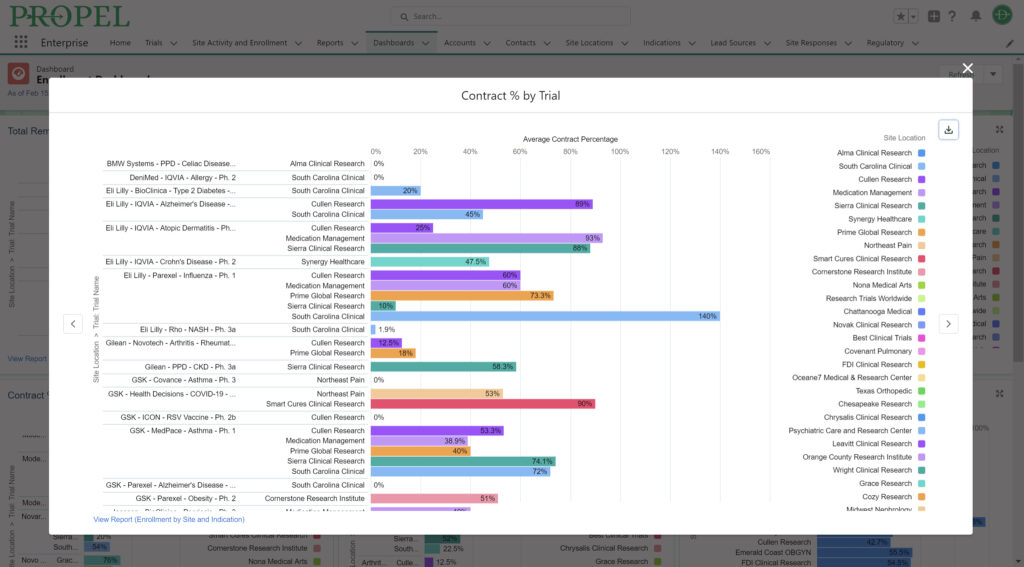
While not the sole determining factor, budget considerations play another significant role in site selection. Sponsors evaluate potential sites based on their proposed budgets, which include personnel costs, overhead expenses, and any additional services required. Additionally, a site’s negotiation skills and their ability to offer flexible contract terms can also influence site selection decisions.
PROPEL further optimizes trial management by centralizing tasks and automating many routine workflows, thereby improving efficiency, reducing the administrative burden, saving time and money, and minimizing errors. The platform’s ability to capture real-time data allows research sites to document these efficiencies and cost savings through detailed reports and key performance indicators (KPIs). This capability helps sites distinguish themselves in a competitive field. Sponsors are drawn to sites that can demonstrate streamlined operations, as it signifies a commitment to excellence and a focus on delivering high-quality data within stipulated timelines.
Communication and Collaboration
Effective communication and collaboration between sponsors and clinical research sites are essential for the smooth conduct of clinical trials. Sponsors further evaluate sites based on their responsiveness, transparency, and willingness to collaborate on trial-related activities. Sites that demonstrate proactive communication and a commitment to addressing sponsor concerns are more likely to be selected as effective collaboration is a cornerstone of successful clinical trials.
PROPEL is purpose-built to facilitate seamless communication and collaboration among site teams, sponsors, CROs, and other stakeholders. Real-time sharing of information, progress updates, and feedback fosters a collaborative environment that instills confidence in sponsors. Sites that leverage PROPEL to enhance collaboration and data-sharing are more likely to stand out to sponsors seeking reliable partners.
Stand Out with PROPEL
Selecting the right clinical research sites is a critical step in the success of clinical trials. Sponsors carefully evaluate sites based on various factors, including experience, patient recruitment potential, facilities, and more. Devana Solutions’ one-of-a-kind PROPEL platform empowers sites to elevate their performance and stand out to sponsors. By streamlining and automating operations, enhancing collaboration, enabling data-driven decision-making, ensuring regulatory compliance, and providing transparent performance metrics, PROPEL equips sites with the tools they need to showcase their expertise and reliability. As sponsors seek out top performing sites to partner with, PROPEL emerges as a key differentiator that enables research sites to shine and win more trial opportunities from sponsors and CROs.
Devana Solutions
A RealTime Software Solutions Company, Devana Solutions is an innovative cloud-based clinical trial software provider that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions even bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.
Read More: Case Study – Accellacare (ICON’s Global Site Network) & Oncacare (specialized oncology site network)