Integrations
Streamline Processes and Increase Efficiency at the Site Level
Eliminate duplicate data entry
Reduce clinical trial cycle time
More robust reporting by trial, site, or organization
Increased therapeutic alignment with Sponsors and CROs
Land more of the “right” trials
Speed therapeutic advances to patients
Devana fully integrates with:



Trial oversight all in one place
Customizable connections with your existing tools
The business of clinical trials requires more than just a CTMS. Devana’s open API gives you the option to integrate with tools your team already uses to manage your current tasks and win future trial awards.

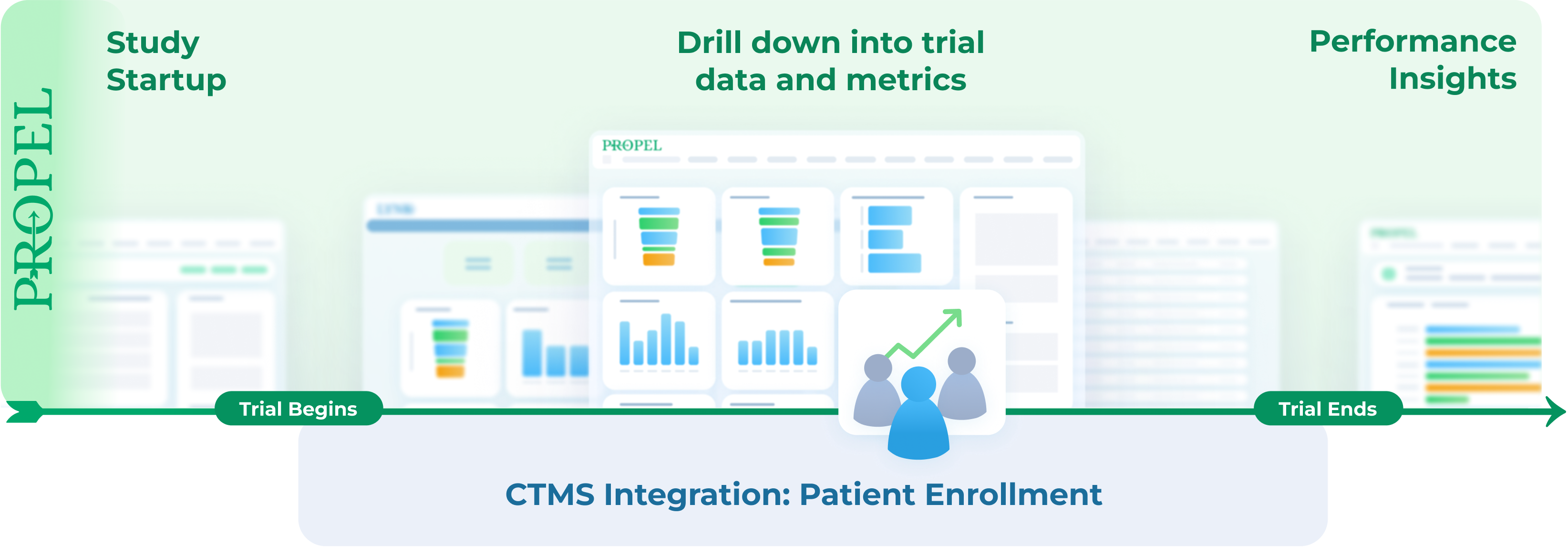
How does PROPEL differ from a CTMS?
A traditional CTMS is designed to manage patient recruitment, scheduling, and study finances. But if you’re only using a CTMS, it can be hard to proficiently track enrollment and site activity, trial opportunities, timing, and performance across your entire clinical trial operations. PROPEL works in conjunction with your preferred CTMS to provide reporting and analytics across the entire clinical trial process. And because your data is securely stored in one place, drilling down into study and site-specific data and performance insights is simple.
|
|
PROPEL |
CTMS |
|
PIPELINE MANAGEMENT AND PLATFORM ADMINISTRATION |
||
|
Pre-Award Workflow Automation |
Start at lead stage and capture data in real time. |
Studies are entered after PSV or Award, so must back date. |
|
Site/Dept. Capabilities and Detailed PI Profiles |
|
|
|
Unlimited Studies |
|
Pay per study. Some offer "template" studies that are not billed until activated, but they are limited in quantity. |
|
Task Management |
Configurable and Automated |
No Automation |
|
Historical Data Capture |
|
|
|
File Storage |
|
|
|
Advanced User Permissions |
|
Varies by CTMS |
|
Unlimited Configuration Across Platform |
|
|
|
Custom Client-Specific Modules |
|
|
|
STUDY STARTUP |
||
|
Track Completion of Milestones |
|
|
|
External User Interface (Portal) for Site Staff |
|
|
|
Automated Email Notifications |
|
|
|
Site Query to Assist with Site Selection |
|
|
|
Trial Sharing and Site Response Capture |
|
|
|
Capture and Track Not Interested and Not Awarded Studies |
|
|
|
CONTRACTS AND BUDGETS |
||
|
Contract and Budget |
High level details and dates. Additional configuration available upon request. |
Store budget with ability to assign values to patients, PIs, etc. |
|
Payment Processing |
- |
|
|
Financial Data Capture |
|
|
|
Budget Build Out and Analysis |
With additional configuration. |
|
|
SCREENING AND ENROLLMENT |
||
|
Patient Database |
- |
|
|
Patient Recruitment |
- |
|
|
Patient Visit Scheduling |
- |
|
|
Patient Enrollment |
- |
|
|
Capture Screening and Enrollment |
Aggregated totals by site, trial, and organization. |
|
|
REPORTING AND ANALYTICS |
||
|
Unlimited Self-Service Reports and Dashboards |
|
Cost associated either for custom reports or reporting tool add-on. |
|
Turnaround Time Performance |
|
|
|
Trial Performance Data: Aggregated or by Site/Dept., Trial, Organization |
|
Only with enterprise systems. |
|
Study Startup Metrics: Aggregated or by Site/Dept., Trial, Organization |
|
|
|
COLLABORATION TOOLS |
||
|
Internal Communication Tools |
|
Varies by CTMS |
|
Email Integration |
|
Varies by CTMS |
|
Surveys (for any use case) |
|
|
|
Time Tracking |
|
|
|
Calendar Integration with Outlook |
|
|
