2023 Year in Review

As December winds down, we can officially say that 2023 has been a year of transformative growth and innovation at Devana Solutions! We’ve seen major updates and enhancements in our product offerings, and experienced exciting developments in our business structure. 2023 has been a landmark year, setting new milestones and paving the way for even greater successes to come.
Products
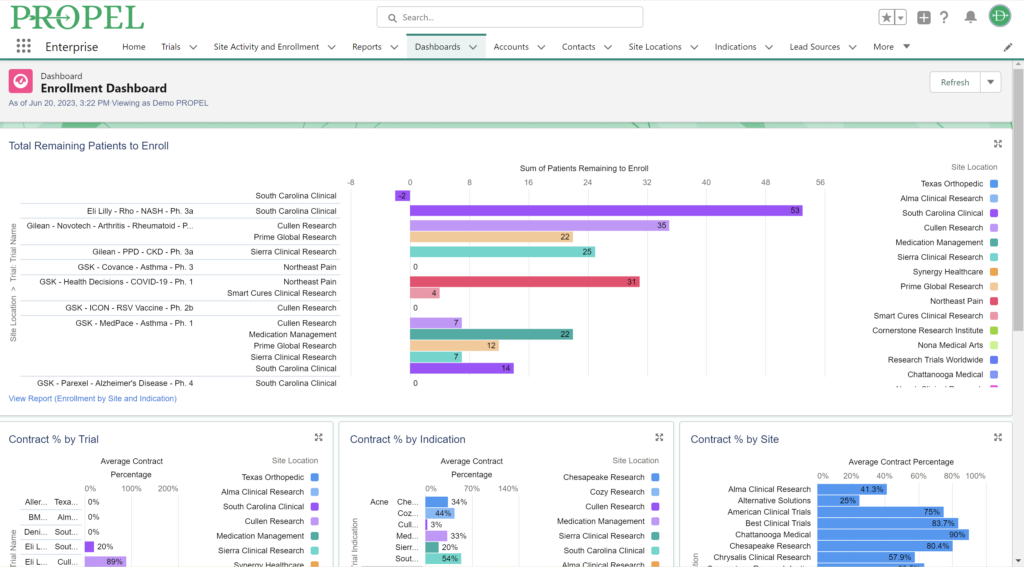
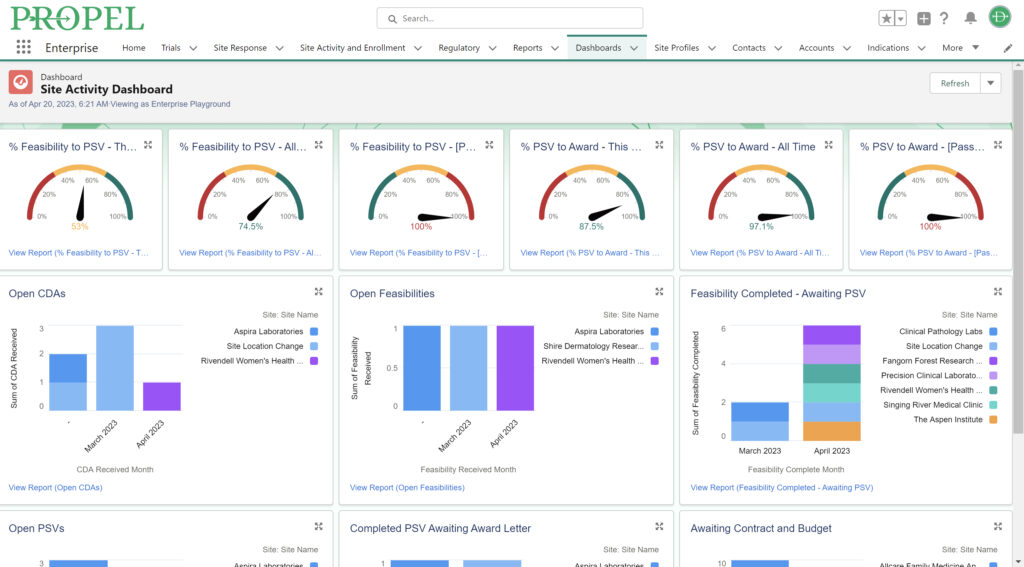
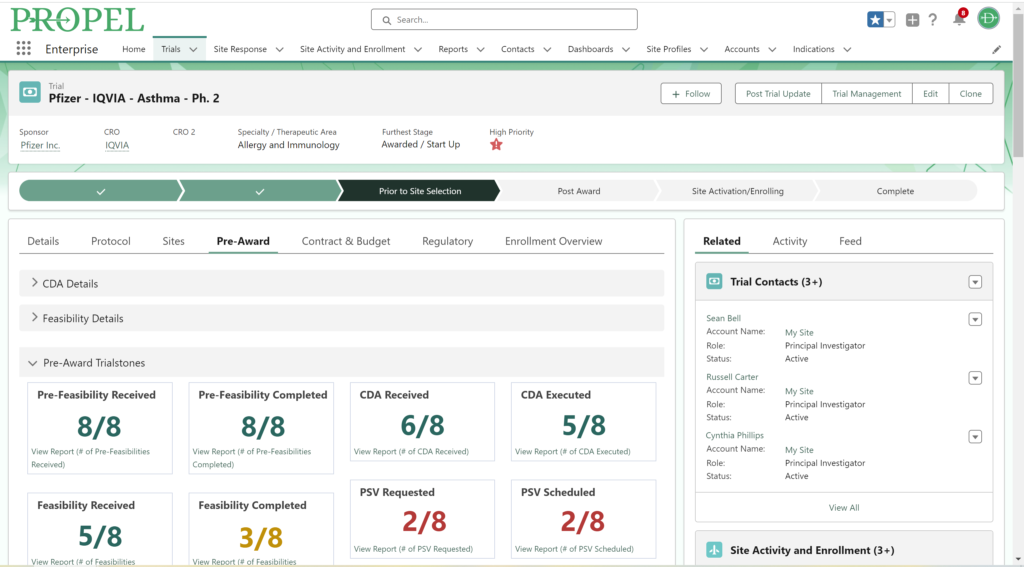
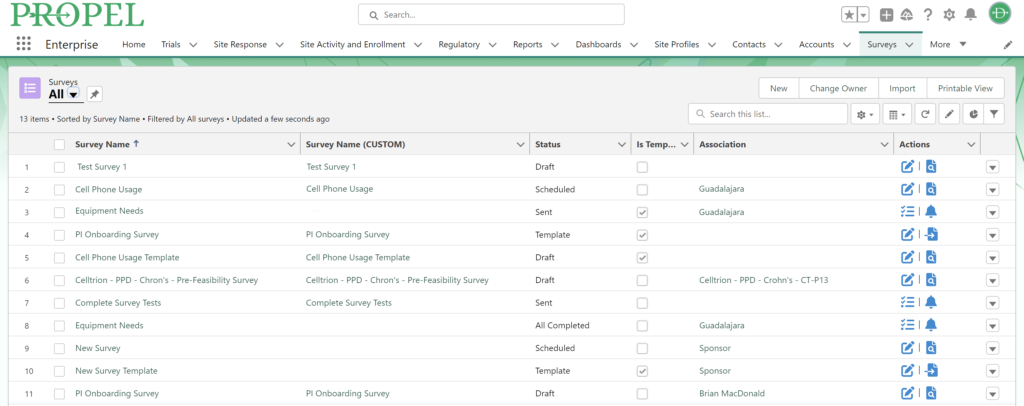
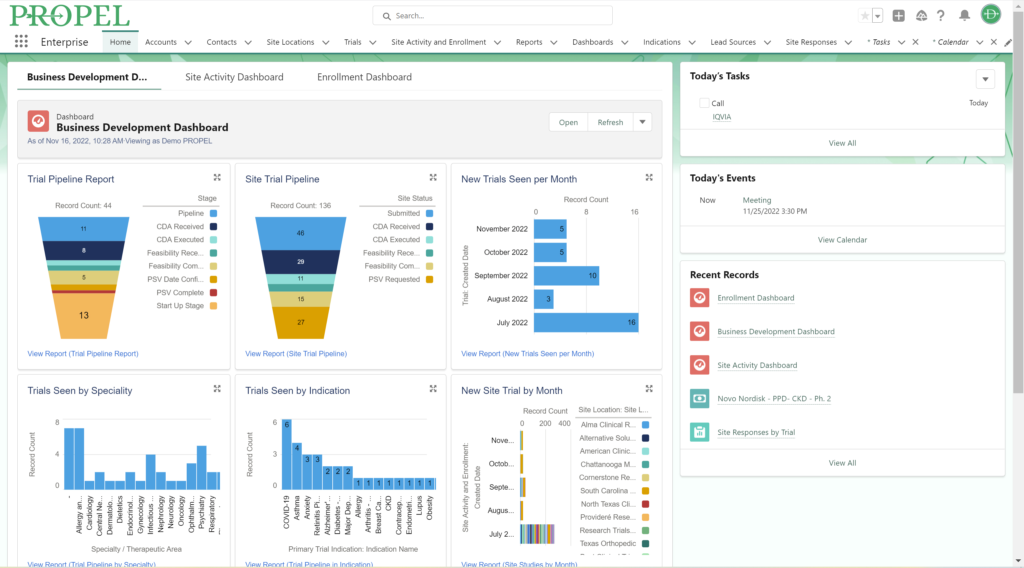
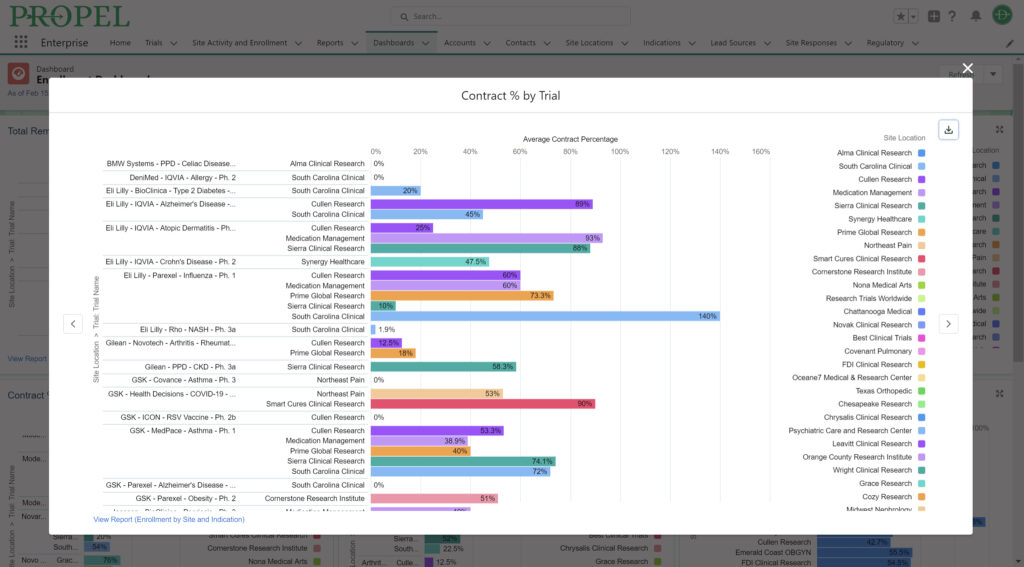
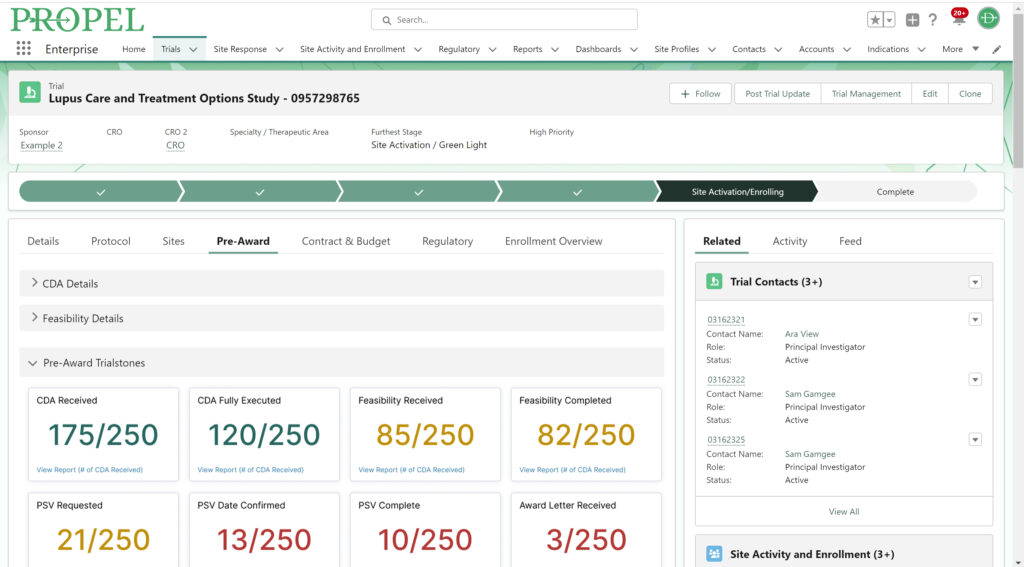
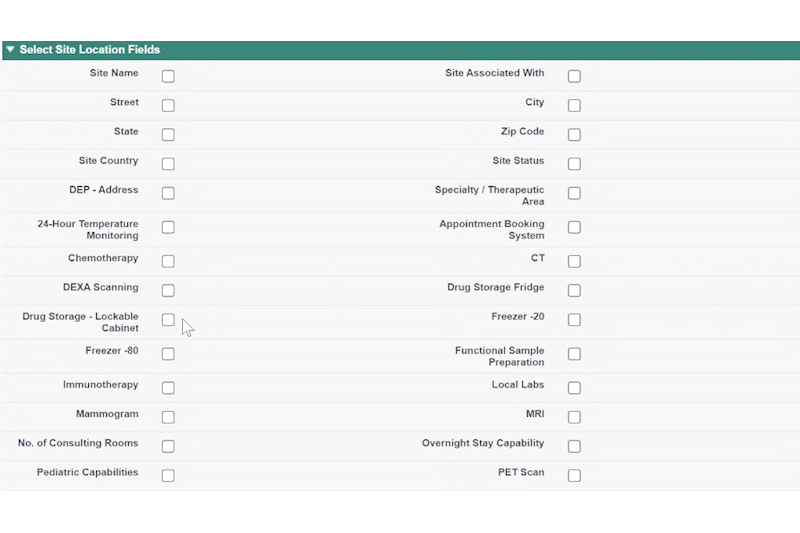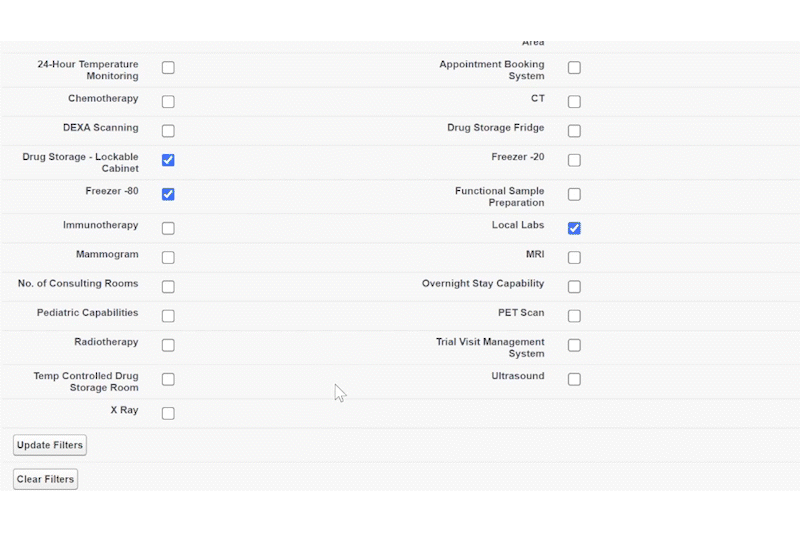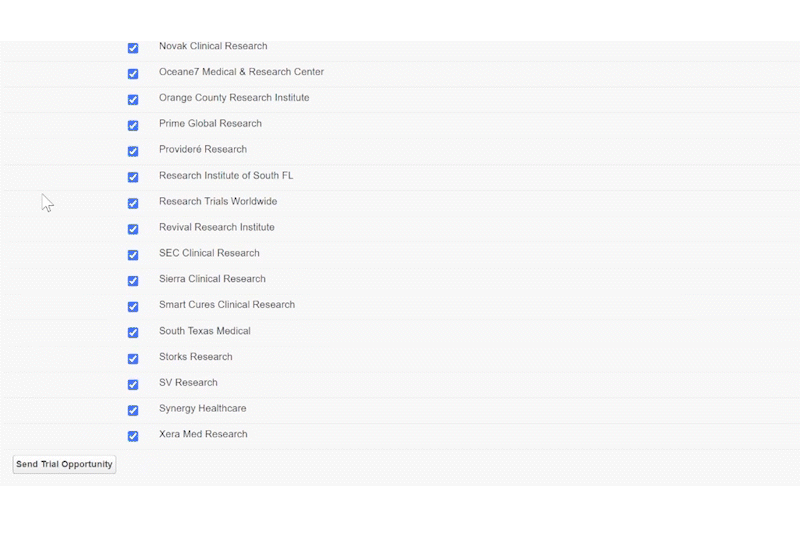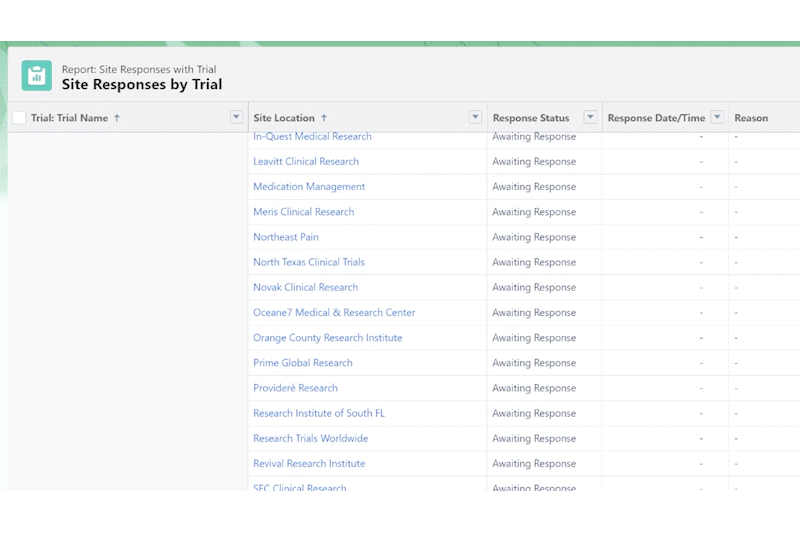
This year, we made a dedicated effort to bring several new interface and usability updates across PROPEL and LYNK. Our category-defining software is now more user-friendly and accessible than ever!
Some of the exciting new features released this year include:
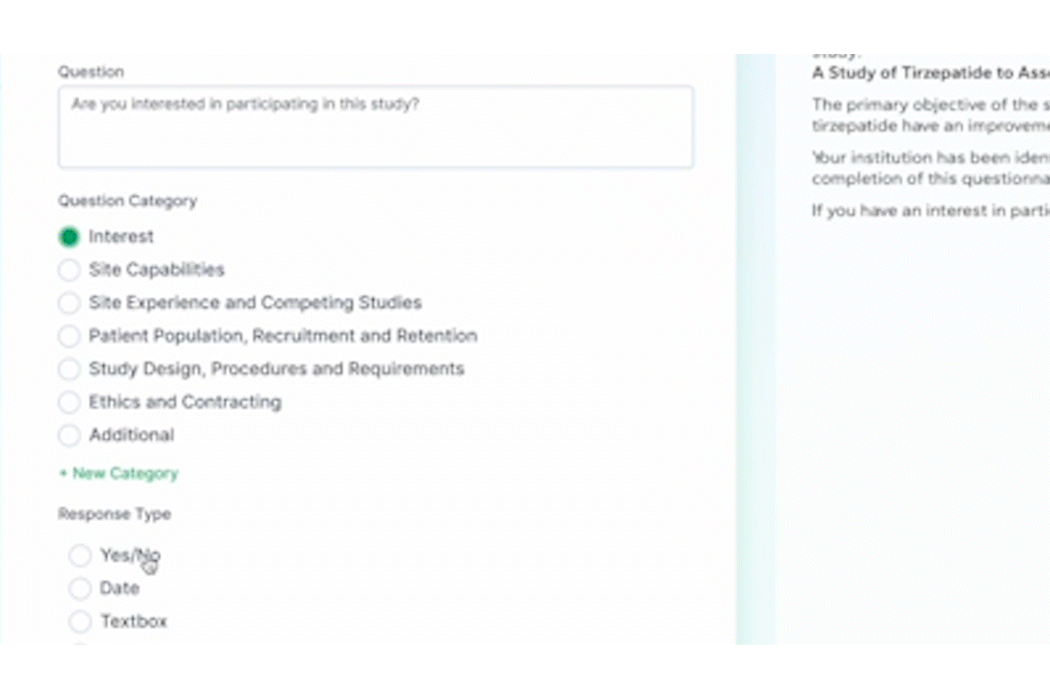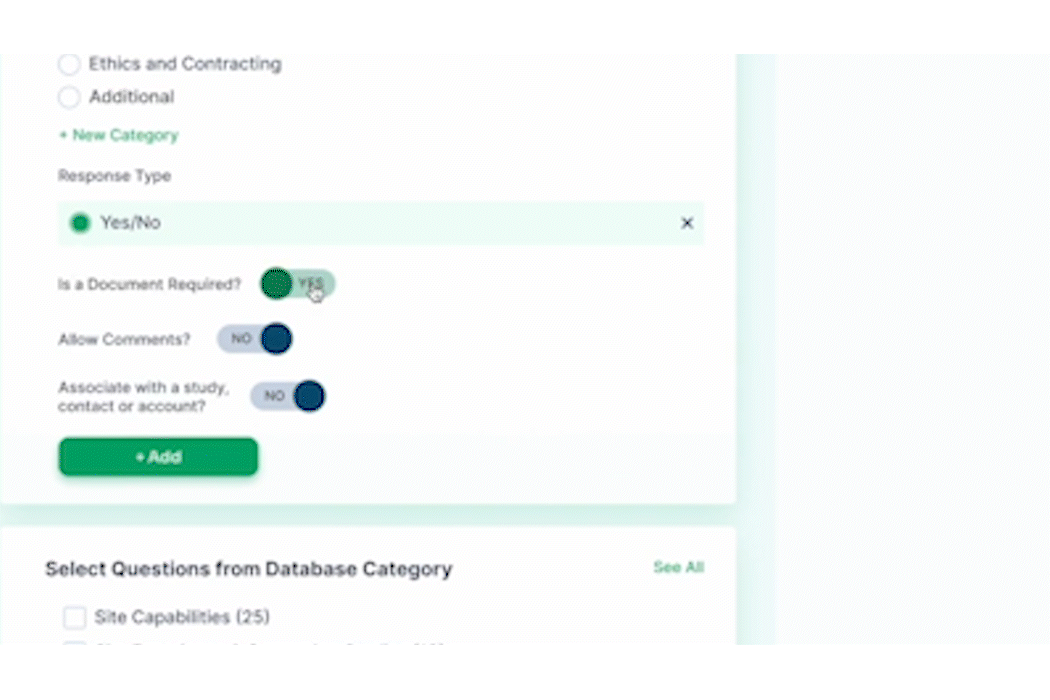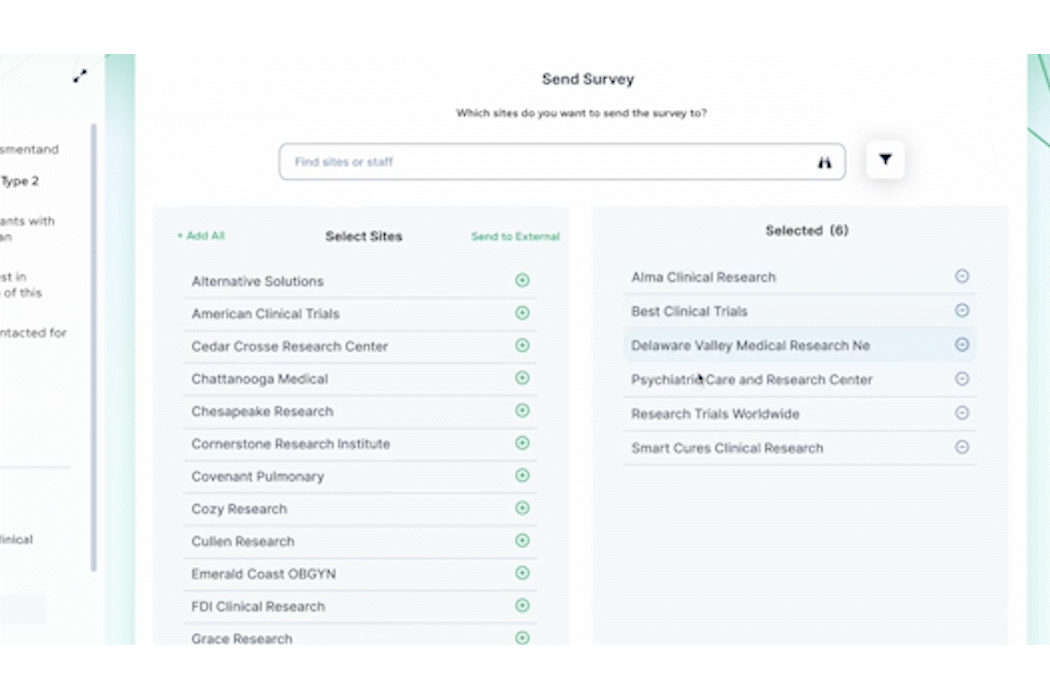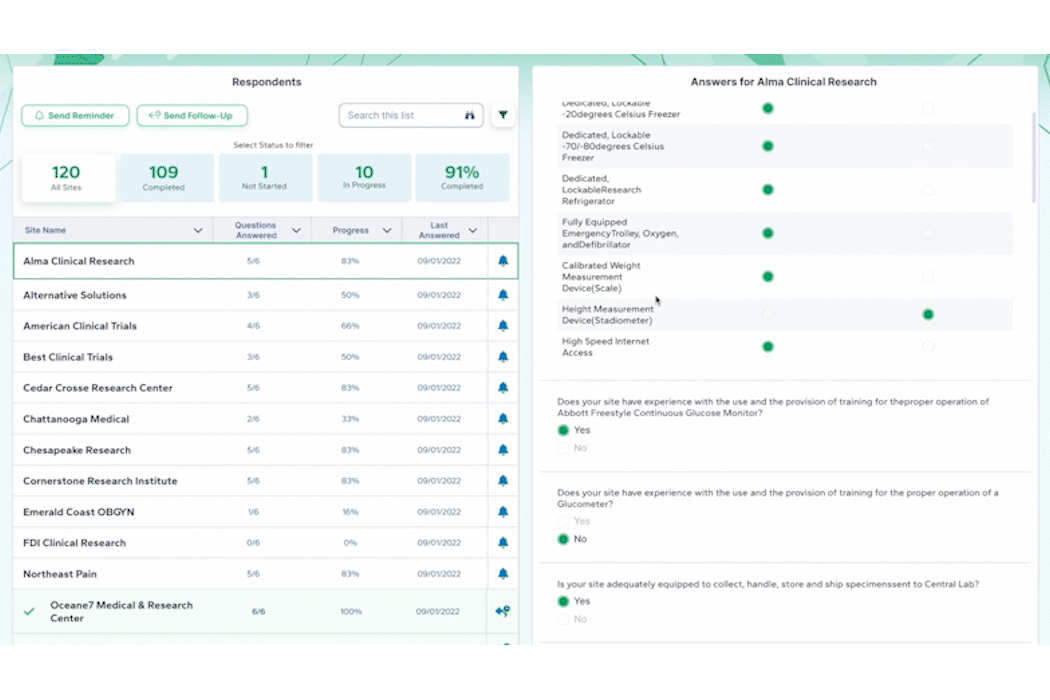
- Surveys
- Notes
- Revenue forecasting
- Trial management hub
In addition to these new items, we also enhanced several existing features and functions of the platform such as trial tracking, document management, historical enrollment metrics, and more. We plan to continue bringing even more features and refinements to PROPEL and LYNK in the future, so look forward to regular updates throughout 2024.
Client-Partners
Customer satisfaction is core to our mission, and we are committed to providing the best-in-class customer experiences. This year, Devana welcomed many new partner-clients to the platform while also experiencing steady growth with existing customers. We are proud to support our partner-clients as they continue to grow and expand their business and site networks across the industry. Such aggressive, sustained growth is only possible with a powerful platform like PROPEL, which provides comprehensive trial management and data analytics for site organizations of all shapes and sizes. We are honored to support them in our shared mission of bringing new and better therapies to patients all over the world.
Giving Back
Alongside our continued partnerships with clients, Devana is also pleased to have continued our support of Greater Gift in 2023. As part of their mission to increase awareness of clinical trials and celebrate those who participate in them, Greater Gift works with other non-profit organizations to pay forward the contributions of individuals involved with clinical research to children in need. This year, Devana ramped up our support of Greater Gift with a new process of making a donation for each new client who completes onboarding with PROPEL. With this method, we now support Greater Gift all year long in addition to our annual donation around the holidays honoring of all our current partner-clients.
Strategic Partnerships
Last, but certainly not least, we want to highlight our partnership evolution with RealTime Software Solutions. Since Devana’s acquisition by RealTime in July, both companies have been hard at work integrating our teams, products, and services. Our companies are aligned in our commitment to customer service and innovation. We are dedicated to delivering powerful solutions that empower the clinical research industry and further streamline the development of life-changing treatments. We’re thrilled to set a new standard for end-to-end site platforms.
What Lies Ahead
As we close the chapter on an extraordinary 2023 and look ahead to 2024, we’re filled with anticipation for the continued evolution of our services, products, and partnerships, all aimed at empowering the clinical research industry. Thank you for being a part of our 2023 journey. Here’s to a new year filled with more milestones, achievements, and shared successes!
Devana Solutions
Devana Solutions is an innovative cloud-based clinical trial software company that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions even bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.