New PROPEL Survey Feature
In the fast-paced world of clinical trials, feasibility surveys have become a crucial step in the process. These surveys help researchers determine the viability of their trials and ensure that all the necessary resources and capabilities are in place. However, it is important to recognize that surveys have the potential to be powerful tools in various other aspects of clinical research. Building relationships, understanding site capabilities, and gathering feedback are just a few of the areas where surveys can prove to be invaluable.
Surveys are an incredibly powerful tool, allowing researchers to collect feedback and insights from a wide range of recipients. Whether it’s gathering information from sites within your network, potential vendors, patients, or investigators, surveys can provide valuable data that can inform important decisions.
PROPEL Enterprise’s new Survey Feature is an exciting development for the clinical trial industry. With this innovative tool, researchers can create and distribute surveys directly within the platform, reaching a large audience and gaining valuable data in a streamlined and efficient manner.
How It Works
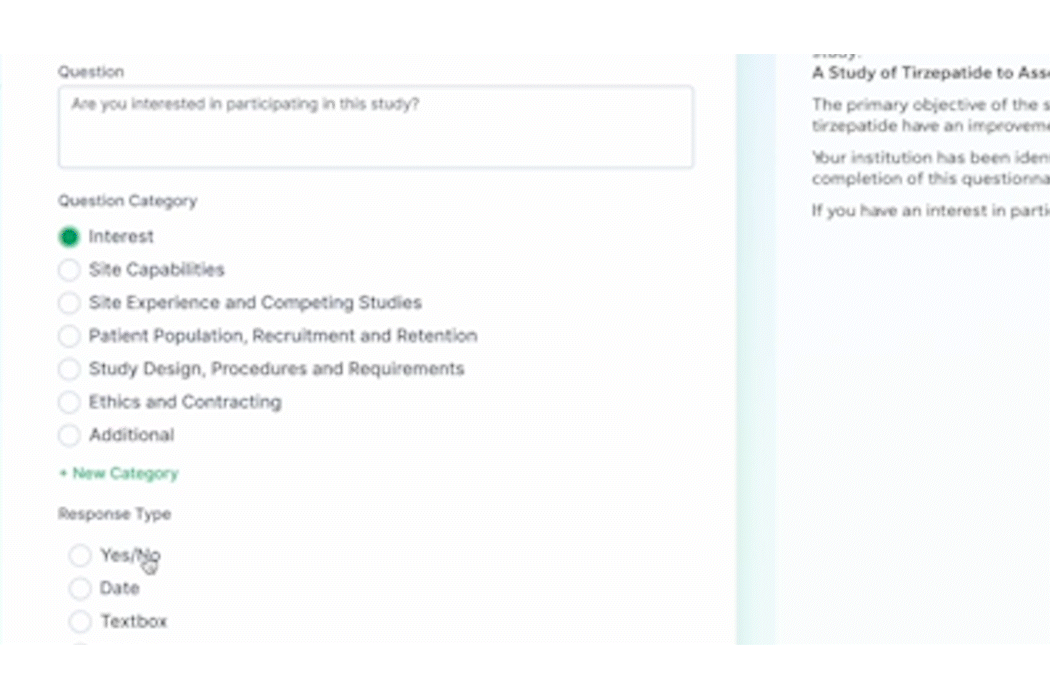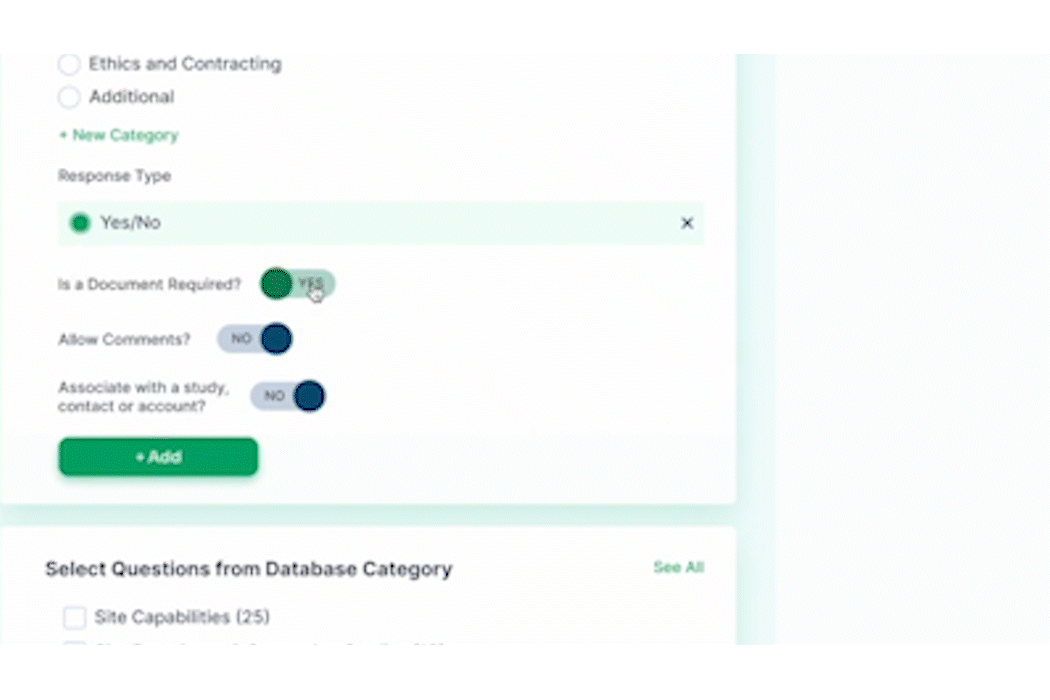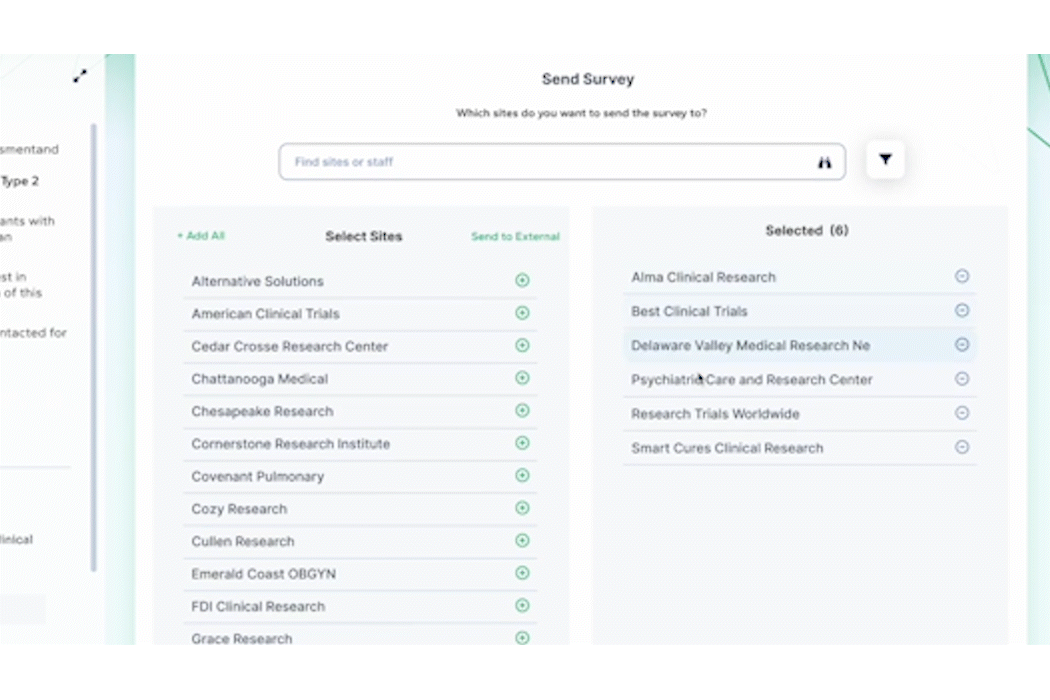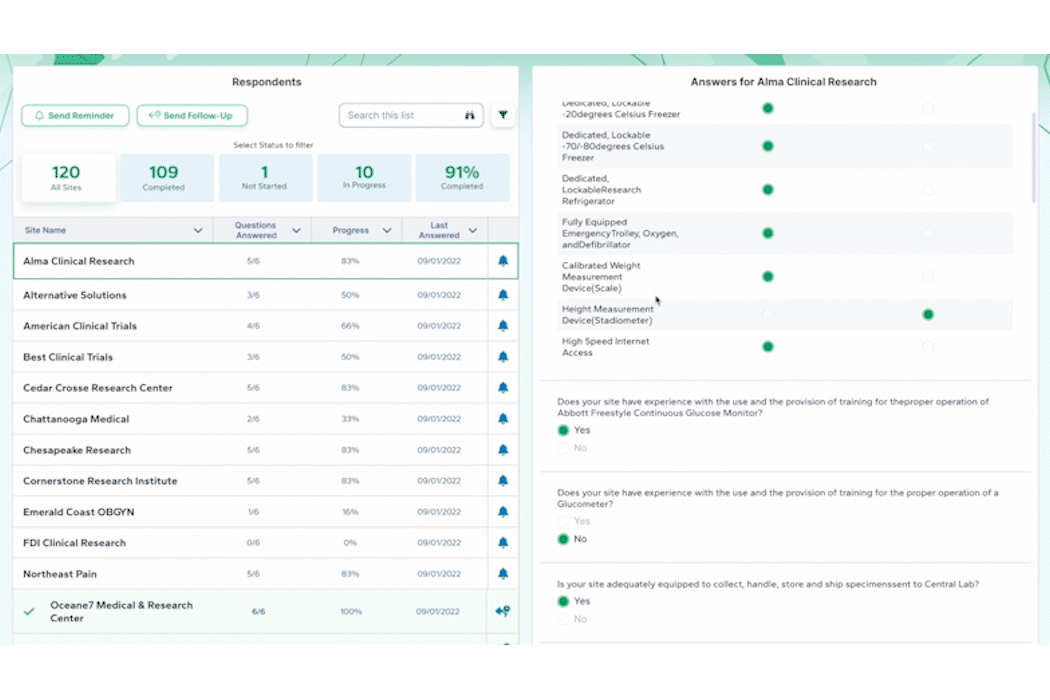
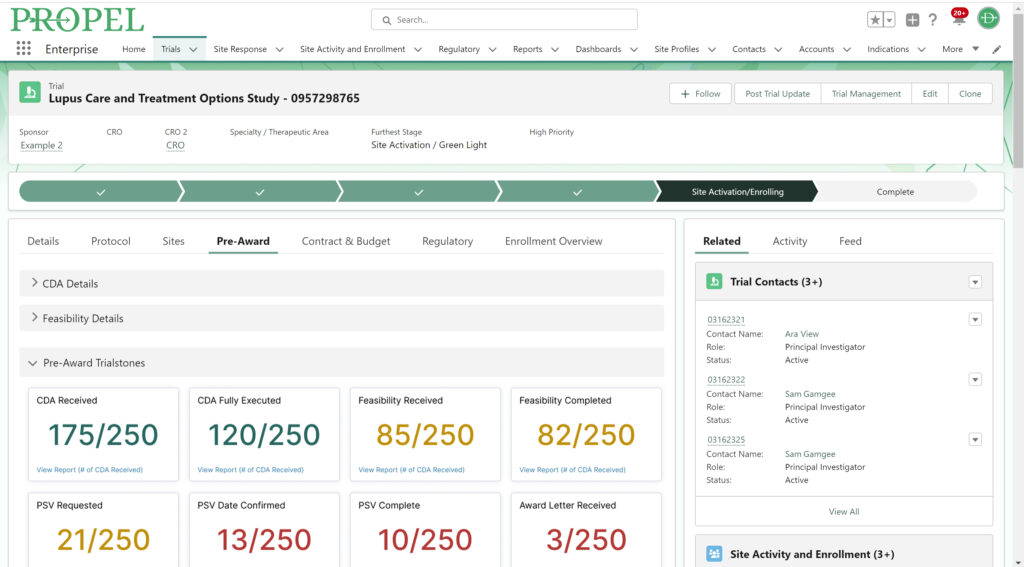
PROPEL Enterprise‘s new Survey Feature functionality (GIF).
Quickly create and send surveys directly within PROPEL to LYNK users or external email lists. Use pre-built templates, import existing questions, or create a survey from scratch with PROPEL’s easy-to-use interface. For ease of tracking, you can also associate your survey with a specific trial, contact, or account.
Once a survey is sent, PROPEL will automatically collect all timing and response data in one place for easy monitoring and reporting – giving you real-time visibility into who has completed a survey, who hasn’t, and who stopped part-way through. But the feature doesn’t stop there – eliminate endless follow-up emails with automated alerts and reminders.
Instead of relying on multiple programs and websites to send and track surveys, PROPEL provides a closed-loop system allowing you to make surveys, send them out, and to get in-depth analytics on responses and timing.
For Any Kind of Survey
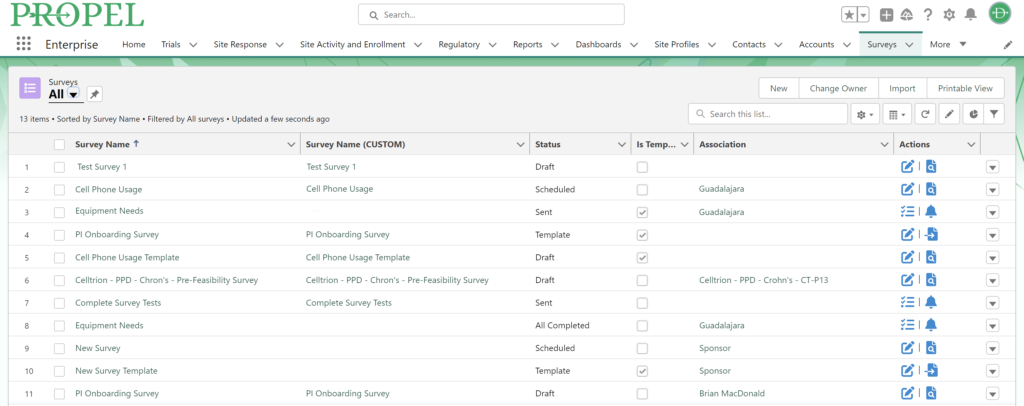
Survey Feature interface.
PROPEL’s new feature aims to transform and streamline the survey process – from feasibility and CSAT to PI onboarding, equipment management, and beyond! Surveys can be as general or specific as you want and for any use case under the sun. Want to know what kind of studies sites are looking for? Need to follow-up with a site or PI about a recent onboarding? PROPEL’s new Survey Feature is here to help!
Ready to see how Devana Solutions continues to revolutionize how clinical research professionals gather and analyze feedback? Schedule a demo today!
Devana Solutions
Devana Solutions is an innovative cloud-based clinical trial software company that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.