Why Your Clinical Research Site Needs More Than a CTMS
Clinical trials require precise management across various domains such as patient recruitment, data collection, regulatory compliance, and stakeholder communication. While Clinical Trial Management Systems (CTMS) have been pivotal in organizing and simplifying many of these aspects for clinical research sites, the complexity of modern clinical research often necessitates a broader array of solutions.
CTMS platforms are instrumental in managing the logistics of clinical trials. Its primary functions include organizing and tracking study milestones, managing patient recruitment and data, ensuring compliance with regulatory standards, and handling site management and financial aspects of the trials. These systems are crucial for maintaining an organized workflow and ensuring that all trial aspects are conducted according to protocol and within regulatory bounds. However, the landscape of clinical research is continuously evolving, driven by technological advancements, increasing data volumes, and more stringent regulatory requirements. As a result, clinical trials are becoming more complex and diversified, often involving multifaceted data sources, varying patient demographics, and innovative study designs. Accordingly, relying solely on CTMS may fall short of meeting the diverse and expanding needs of modern trials.
Beyond enrollment and the more limited scope of a CTMS, Devana Solutions’ PROPEL cloud-based platform expands the power of your data and trial administration. From pipeline management and pre-award milestones through to trial completion, PROPEL enables you to standardize, streamline, and automate all of your clinical trial processes.
Enhanced Data Analytics
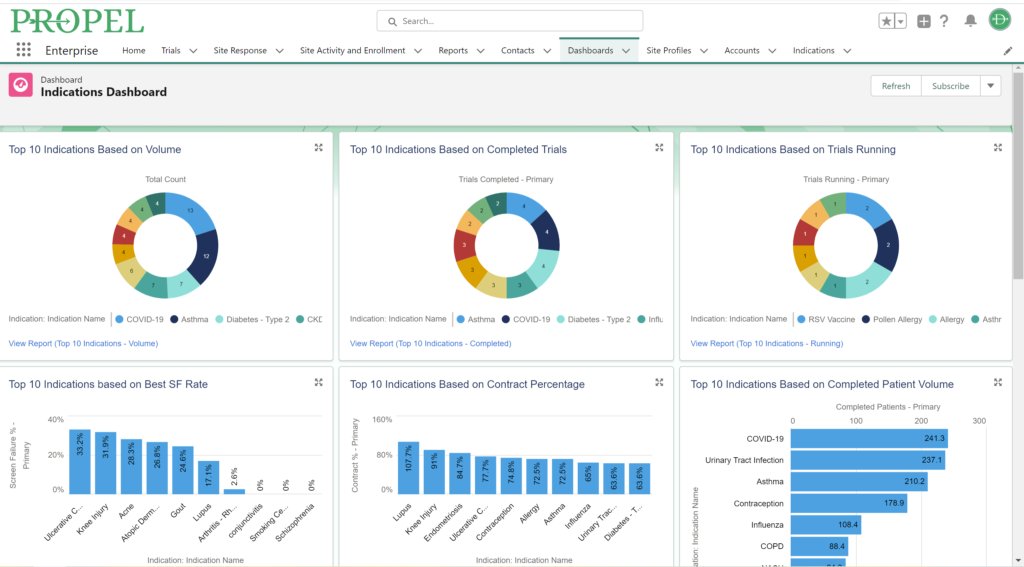
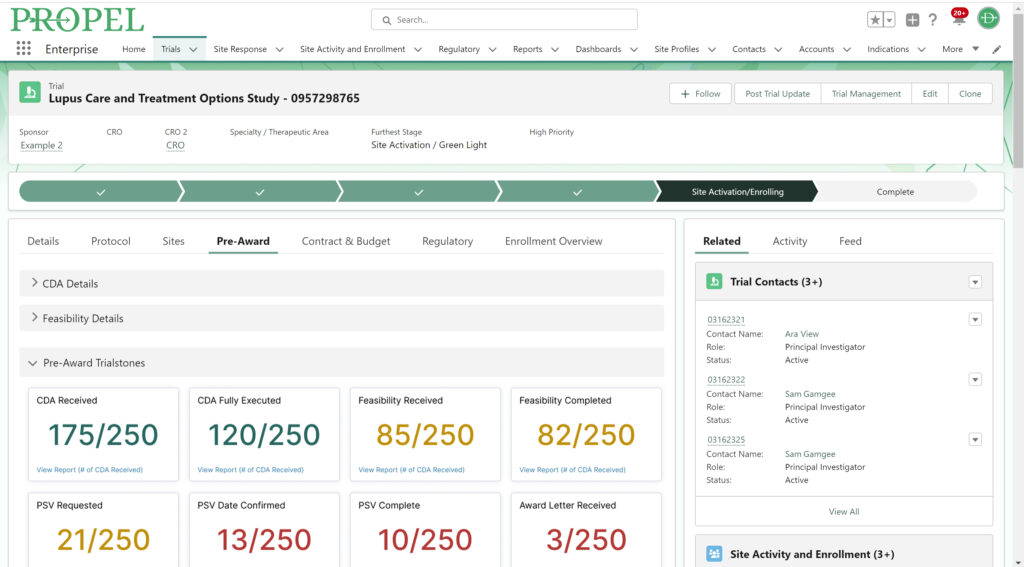
PROPEL Indications Dashboard.
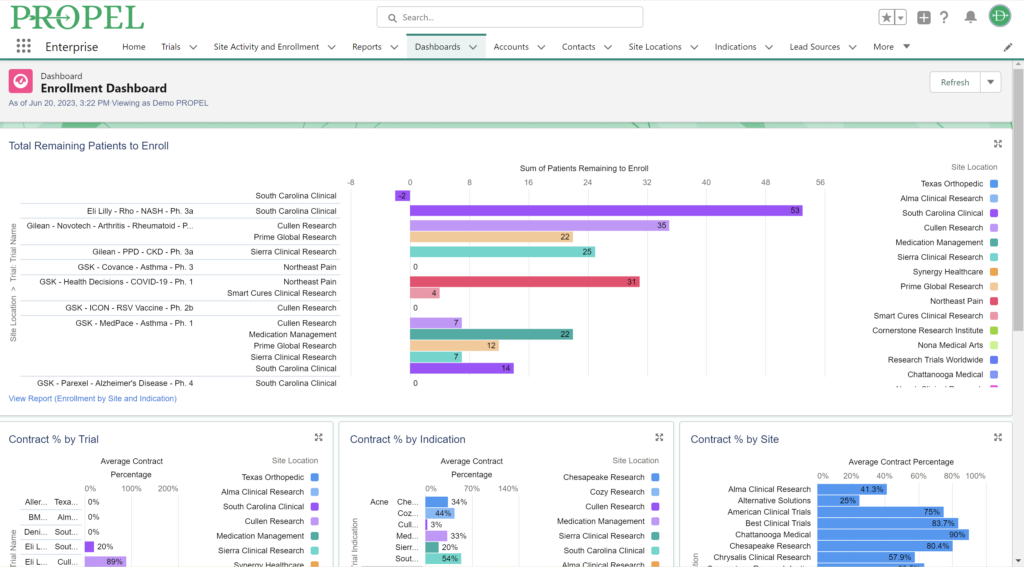
Clinical trials generate vast amounts of data, and leveraging this data effectively can be a game-changer. While a CTMS is proficient at managing enrollment and select operational data, PROPEL adds a layer of advanced analytic capabilities on top. PROPEL’s seamless integrations with the leading CTMS providers eliminate duplicate data entry and offer more robust reporting. See all your data for the entire clinical trial process in one place. From opportunities still in the pipeline to trials in pre- and post-award stages, easily break down your data by trial, site, indication, sponsor, and more – all in real time.
In combining PROPEL’s analytics with a CTMS, research organizations gain valuable insights into site and overall performance, allowing for data-driven decisions that optimize trial design, recruitment strategies, and resource allocation. Plus, PROPEL’s powerful collaboration features allow you to seamlessly incorporate site feedback and updates into decisions, ultimately improving overall patient experience and trial outcomes.
Adaptive Trial Management
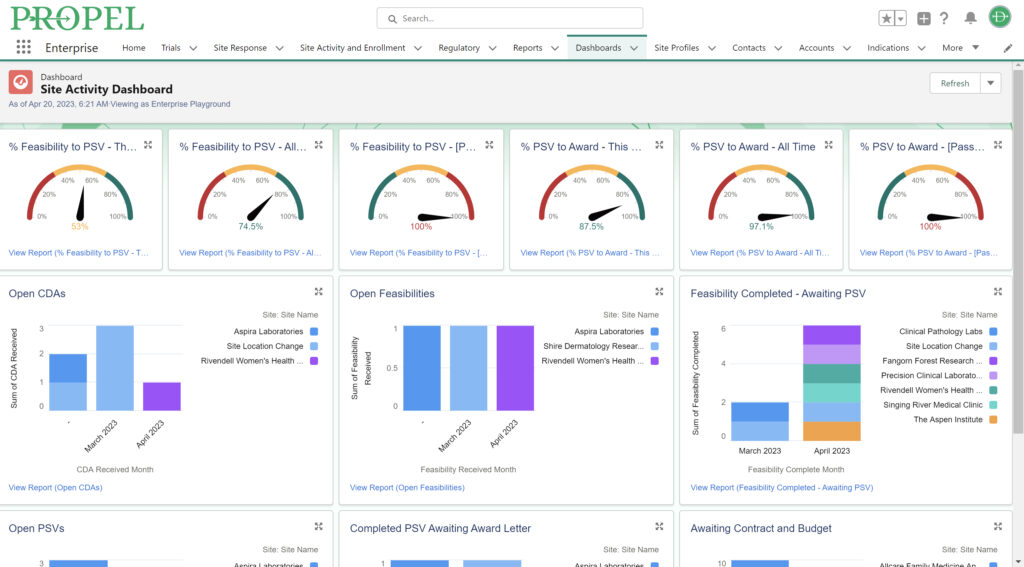
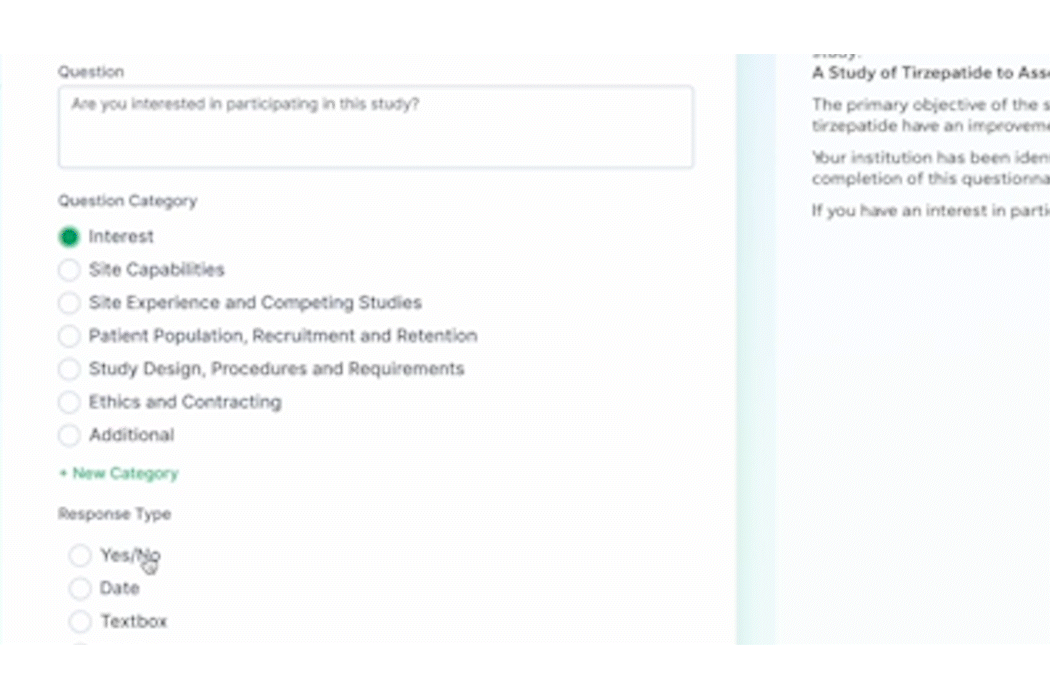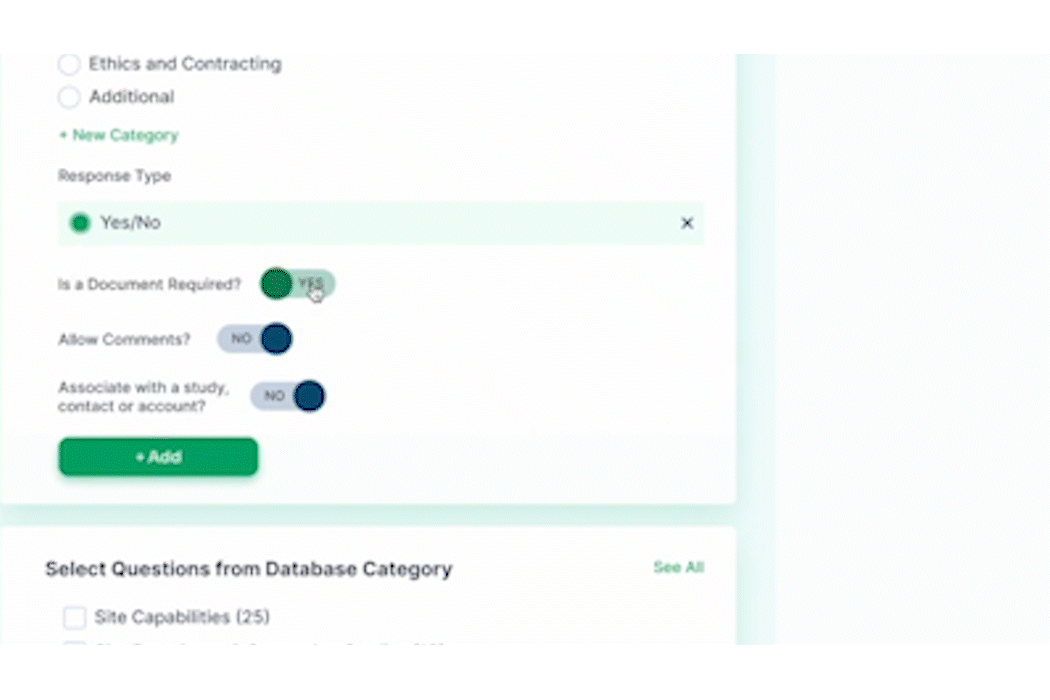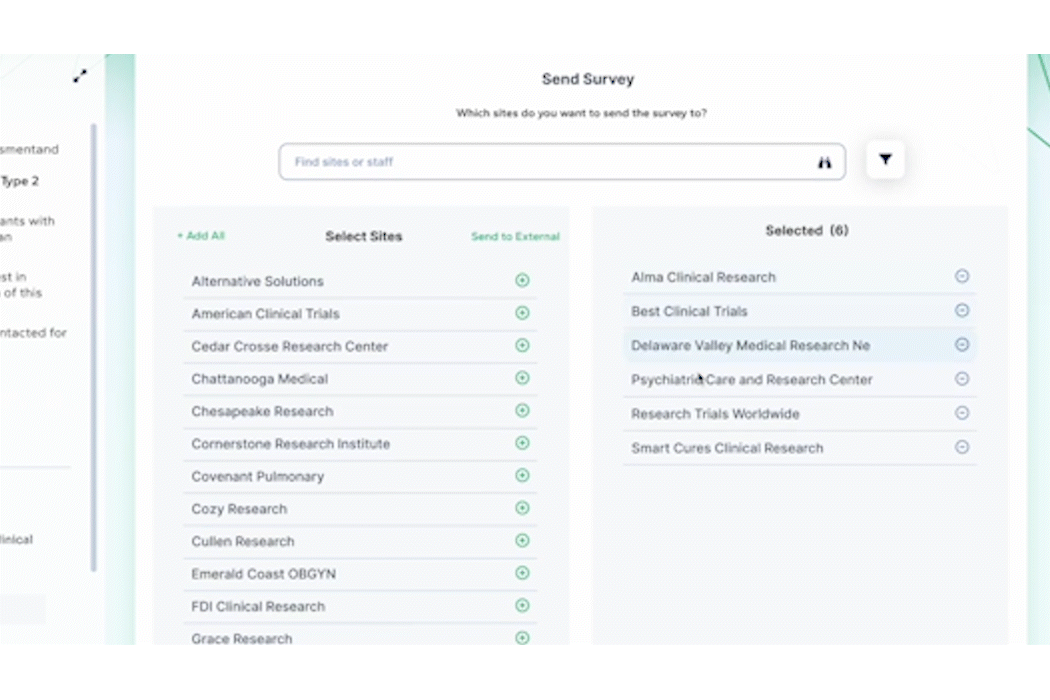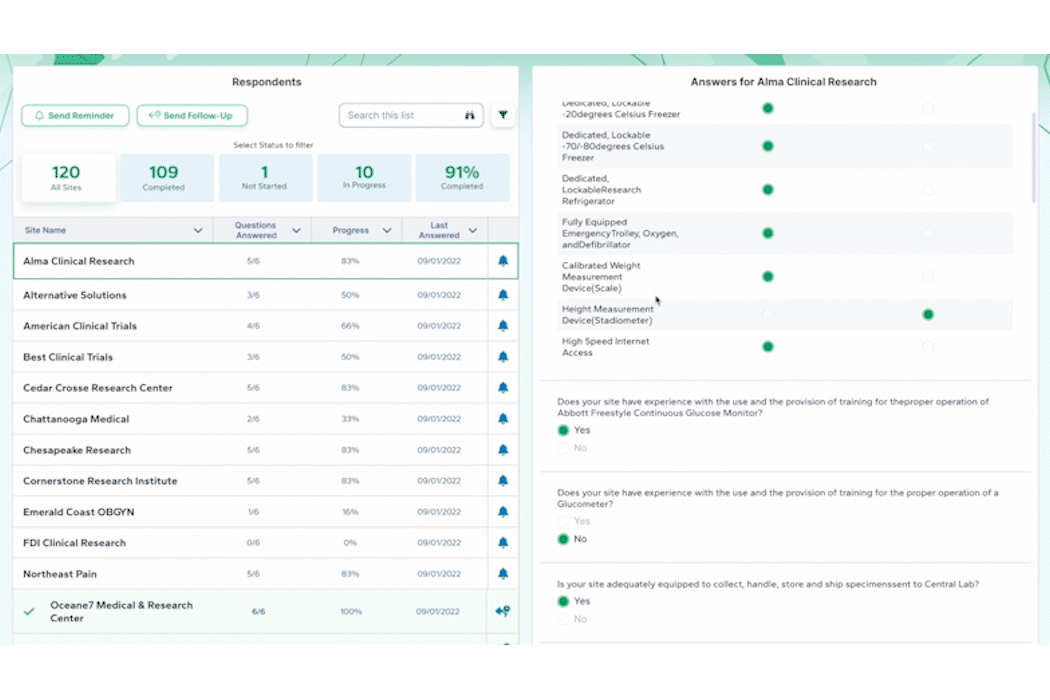
In clinical trial management, there is an increasing focus on adaptive trial designs and real-time adjustments. An adaptive design adds flexibility, allowing for certain modifications to be made after a trial is underway. The FDA released guidance on such trial designs in 2019. PROPEL’s agile project management tools and focus on site engagement complement a CTMS by providing a dynamic approach to trial management. With automated study startup workflows, PROPEL allows organizations to standardize and accelerate processes – saving time and money while also providing full visibility into current and next steps.
Once a trial begins at a site, tracking progress and staying in touch is made easier with PROPEL – allowing both sides to update milestones, share key documents, and respond to questions. As a trial moves through the system, PROPEL captures turnaround timing metrics and performance insights which can be used to identify patterns and make quick adjustments on the fly.
By integrating PROPEL with a CTMS and empowering sites, research organizations can adapt to emerging challenges, optimize resources, and make informed decisions on site selection and management, ensuring the success of adaptive trial designs.
Beyond CTMS
While a CTMS remains an indispensable tool for managing the operational aspects of clinical trials, its potential is maximized when integrated with complementary solutions like PROPEL. Working together, your CTMS and PROPEL provide a holistic view of your entire clinical trial process from start to finish. Manage trials, contacts, documents, and more – all in one place. And PROPEL’s open API also allows you to integrate other tools your team uses such as Outlook, Pardot, and beyond to help manage your customers and win future awards.
An approach that combines patient-centric technologies, data analytics, regulatory compliance tools, and adaptive trial management systems empowers researchers to navigate the complexities of modern clinical research successfully. By embracing a comprehensive ecosystem of integrated solutions, the clinical trial community can enhance efficiency, improve patient outcomes, and accelerate the development of innovative treatments.
Devana Solutions
A RealTime Software Solutions Company, Devana Solutions is an innovative cloud-based clinical trial software provider that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions even bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.

 Devana Solutions is proud to announce their sponsorship of
Devana Solutions is proud to announce their sponsorship of