Speed Up Your Research Site Organization’s Turnaround Times
Regardless of size and type, clinical research site organizations compete for trial opportunities from CROs and sponsors. There are many factors that can make a site stand out amongst the crowd, but turnaround times for key items may be one of the larger deciding factors. CROs and sponsors want to get trials up and running as quickly as possible and demonstrating that your organization has a history of turning things around quickly can make you stand out.
Designed specifically for the clinical trial industry, PROPEL accelerates workflows and automatically captures and records turnaround timing and performance metrics along the way.
Accelerated Workflows
Speed matters in clinical trials. That’s why PROPEL accelerates every step of the clinical trial process – from pipeline management to study startup and beyond. Optimize execution with standardized processes that offer full visibility into current and next steps across your organization, saving time and reducing the risk of things slipping through the cracks or getting delayed.
Once a trial begins enrollment, it’s hard to speed things up. That’s why it’s important to accelerate those tasks which can be sped up, saving time and manpower for other critical areas of focus such as patient recruitment and enrollment. Designed to streamline every process possible, PROPEL eliminates many repetitive tasks, including data entry into a myriad of systems or sending countless emails to update sites. Instead, PROPEL’s seamless integrations allow you to enter data only once, while real-time collaboration tools foster easy communication with sites. Tracking trial milestones is also made easier, allowing sites and central teams alike to update progress, share key documents, and alert others to any roadblocks or questions.
And, as trials move through the system, PROPEL automatically tracks turnaround times for activities such as business development, study startup, site-based milestones, and more – allowing you to see patterns and make informed decisions.
Process and Performance Transparency
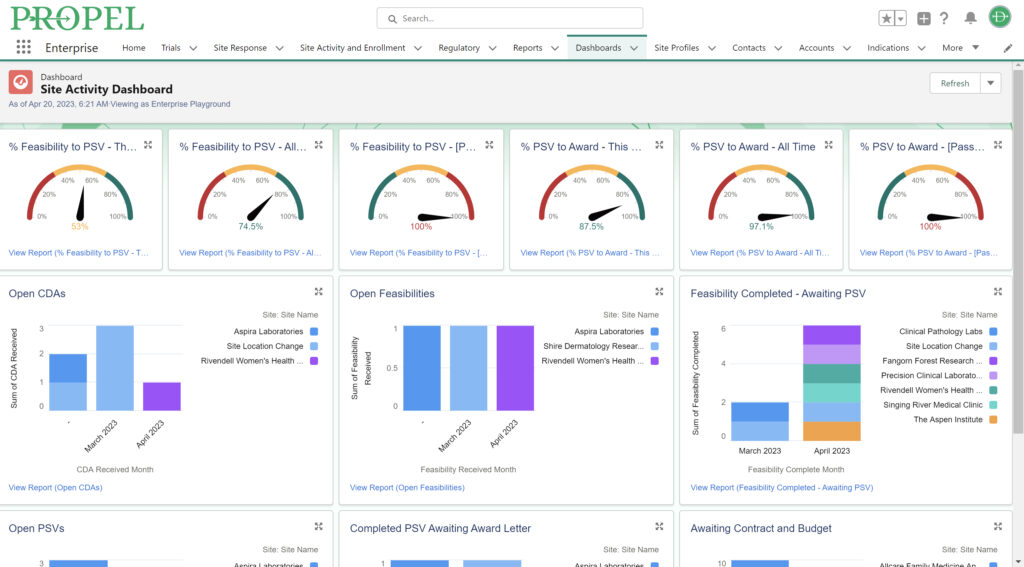
PROPEL Site Activity Dashboard
Data transparency is critical to effective clinical trial management. PROPEL was engineered with proprietary algorithms that automatically capture hundreds of process and performance metrics by indication, site, PI, trial, and more. In fact, every data point within PROPEL is reportable – allowing users to create custom dashboards and reports to analyze and share with key stakeholders.
PROPEL’s cloud-based platform allows central teams to collaborate with site-based study teams in real time. Gain insight into your entire organization’s trial process and data from start to finish as it happens, allowing you to drive buy-in and accountability across the board. Effortlessly monitor the latest metrics on trial status, site activity, patient enrollment, and more – allowing central team members to take swift action based on the most up-to-date site activities and enrollment data, leading to faster decision-making and improved trial outcomes. This data can then improve the overall trial process by matching the right sites and investigators with the trials that best suit their capabilities and location. Once the proper teams are aligned, you’ll experience an increase in trial performance, reducing cycle times and allowing you to better predict future performance.
Improve turnaround times and stand out to CROs and sponsors with PROPEL’s powerful workflow improvements and data analytics. Easily show both real-time and historic reports based on site activity, enrollment, indications, and more – helping win more trials that suit your organization’s strengths.
Devana Solutions
Devana Solutions is an innovative cloud-based clinical trial software company that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions even bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.

 The World Vaccine Congress, in existence for over two decades, has grown to become the largest and most established conference dedicated to vaccines, globally. The
The World Vaccine Congress, in existence for over two decades, has grown to become the largest and most established conference dedicated to vaccines, globally. The