Accelerate the Clinical Trial Process
Clinical trials are essential for advancing medical research and bringing new treatments to patients. However, the complexity involved in organizing and managing these trials can often result in delays and inefficiencies. To overcome these challenges and accelerate the clinical trial process, it is crucial to focus on streamlining efficiency and collaboration among the multiple teams and tools involved.
Purpose-built specifically for the clinical trial industry, PROPEL empowers organizations to manage the entire clinical trial process easily and efficiently – from pipeline and study startup through trial completion and analysis.
Pipeline Management
PROPEL offers a robust platform for pipeline management, allowing Business Development team members to easily input and track new trial opportunities from both CROs and sponsors. By logging calls and emails related to these opportunities, PROPEL provides a comprehensive overview of touchpoints and progress. This not only streamlines the process but also improves visibility for leadership and other team members.
With PROPEL’s powerful dashboards and data management, users can quickly create custom reports and data visualizations to share with key stakeholders. Easily break down past and current trial statistics by indication, site, investigator, and beyond. This powerful data analysis can help your organization win more trial opportunities by showing both historic successes and real-time insights into currently running trials.
Site Selection and Feasibility
Selecting the correct sites for any given trial is an important step of the process. You want to ensure the best possible outcome in terms of enrollment, timing, and overall results. PROPEL’s precision query feature allows you to select the most appropriate sites based on both site and investigator criteria, including capabilities, equipment, therapeutic specialties, and more. Once the best sites have been selected, you can send the trial opportunity directly within the platform and receive real-time responses in return. PROPEL’s threaded chat conversations allow central and site-based teams to correspond back and forth – eliminating the need for follow-up calls or emails to field questions and share documents. As sites respond to opportunities and begin the pre-award process, PROPEL automatically records their responses and turnaround timing metrics.
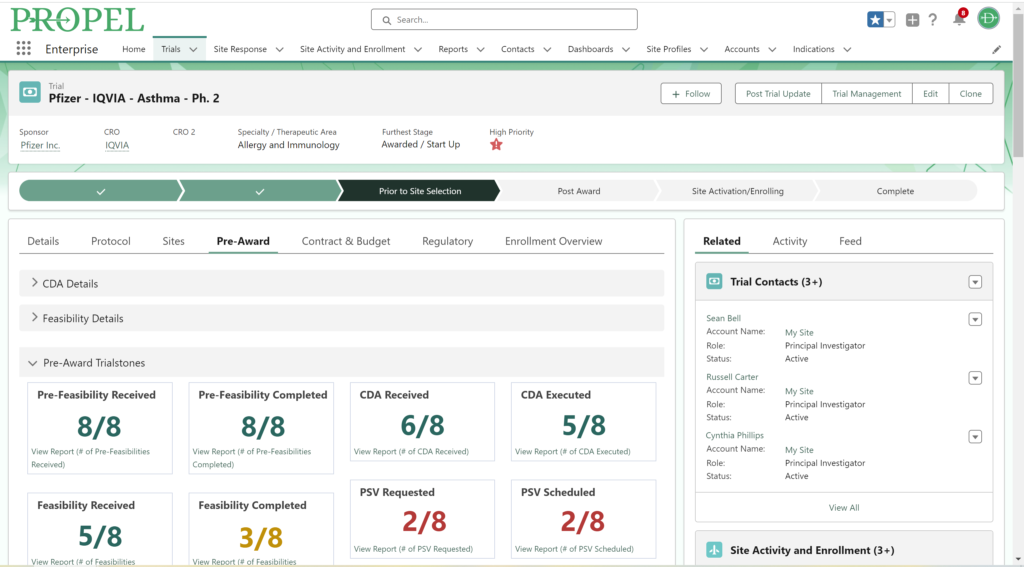
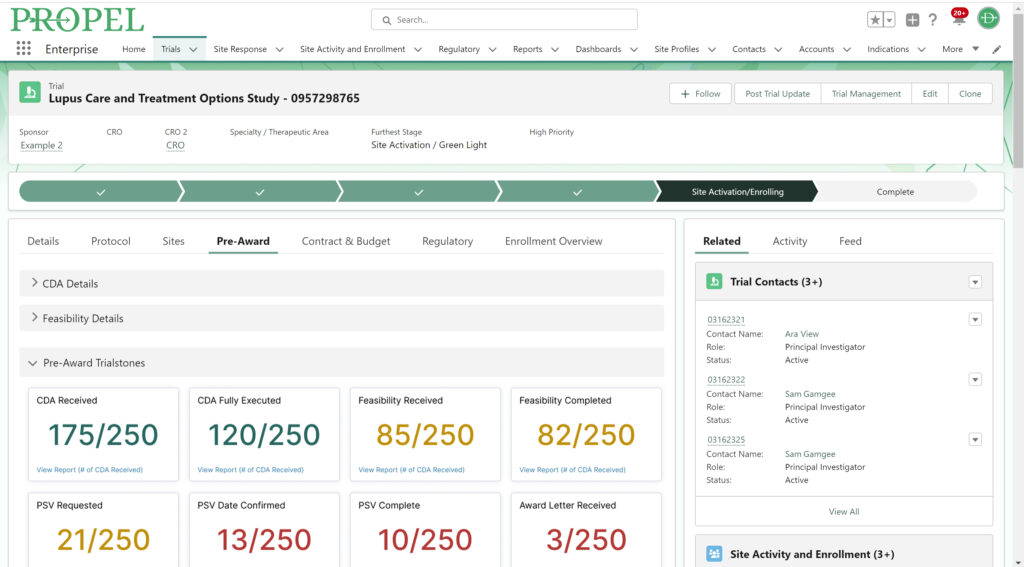
PROPEL Pre-Award Trialstones
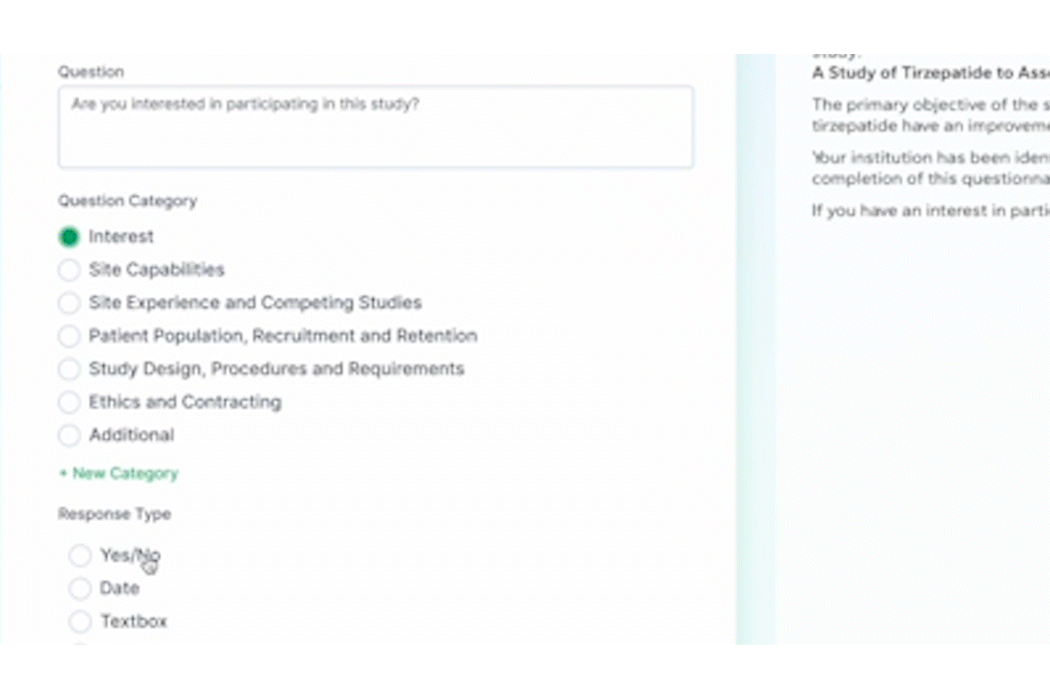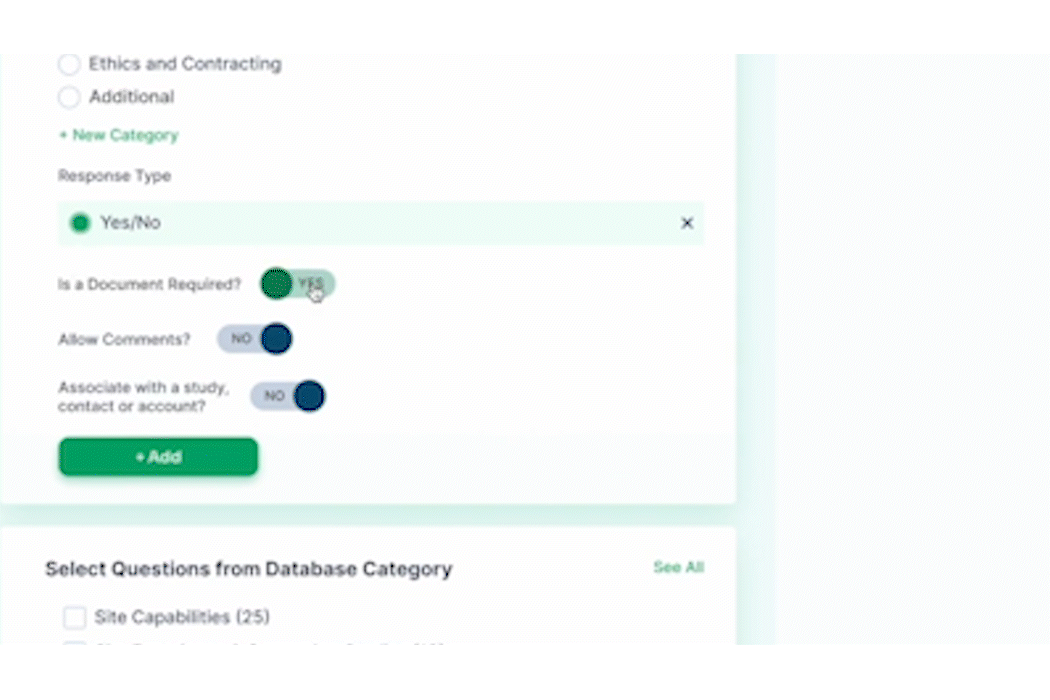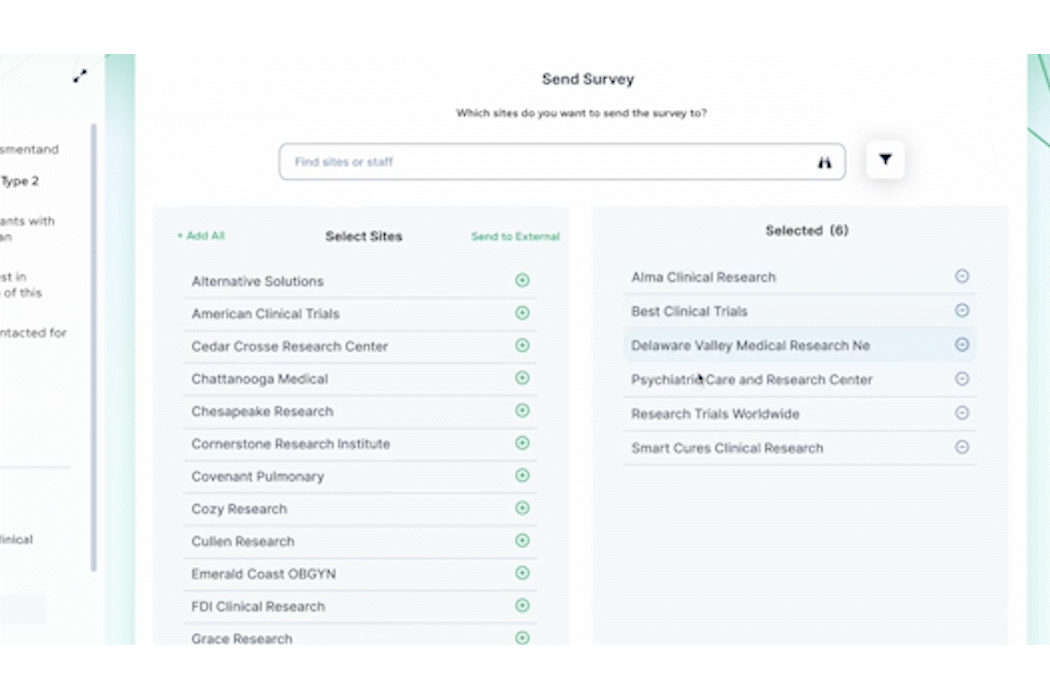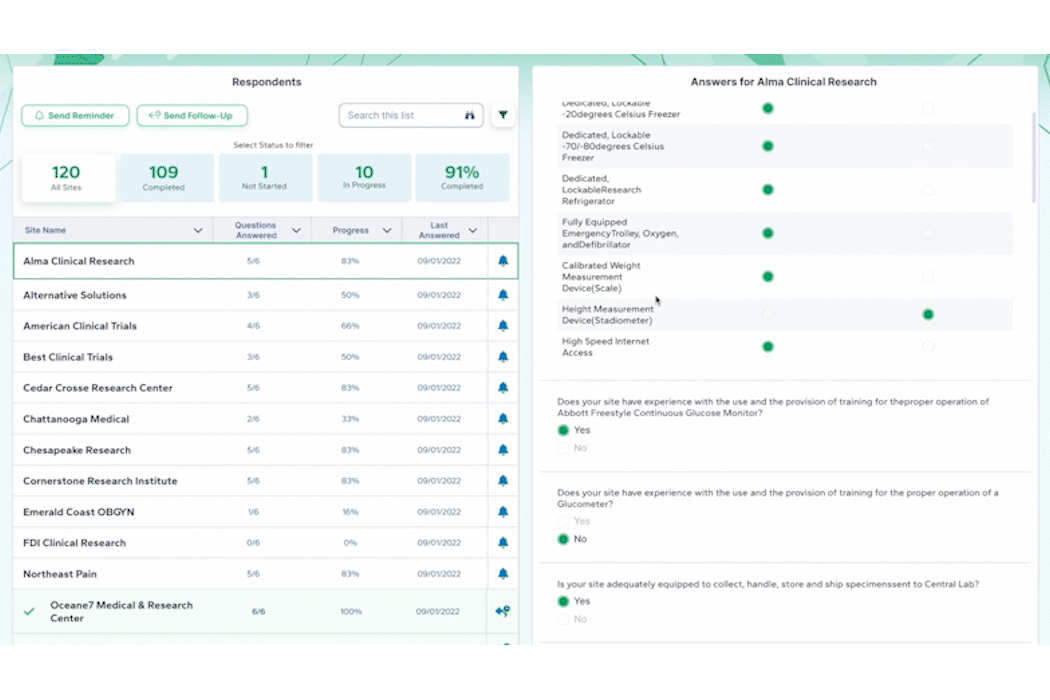
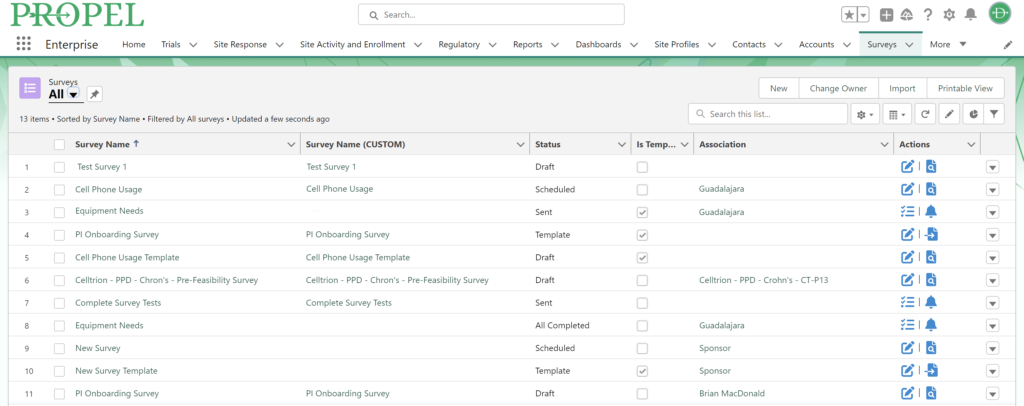
As the pre-award process begins, PROPEL tracks site milestones as they progress through the stages. Using PROPEL’s Survey Feature, Feasibility team members can create and distribute pre-feasibility and other types of surveys to sites. Once sent, they have real-time visibility into who has completed a survey, who hasn’t, and those who stopped midway. Monitor responses and send custom reminder alerts to prompt sites to complete open surveys. Based on responses, trials can continue to progress at the most suitable sites.
Study Startup
Once a trial is awarded to a site, PROPEL makes it easy to follow their progress with improved trial management and monitoring. Track site activity and key post-award milestones including contract, budget, and regulatory stages as sites progress through the study startup process. Central team members can assign custom tasks and alerts to specific users to keep things moving smoothly whenever they see a slowdown or bottleneck.
To reduce headaches and improve workflow, PROPEL keeps all trial-related documents and communication in one place. Documents such as budget and protocol amendments can be attached to specific trials or site activity records, and central team members can send out updates and alerts directly within the system. Real-time collaboration between central operations and site-based staff is made easy using PROPEL’s chat feed or linked email correspondence. Keep everyone in the loop on the latest updates while also making communication trackable and reportable.
Screening and Enrollment
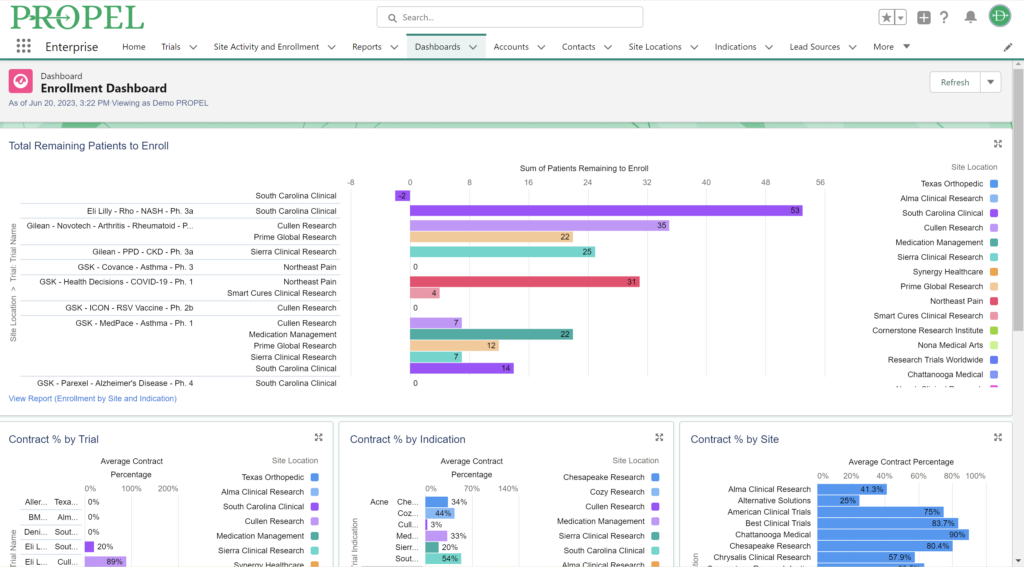
PROPEL Enrollment Dashboard
Upon site activation and the beginning of patient enrollment, PROPEL continues to track progress by integrating with the leading CTMS solutions to monitor site enrollment. PROPEL works in conjunction with your preferred CTMS to eliminate duplicate data entry and provide total trial oversight. Operations and Enrollment team members can monitor enrollment progress across all sites and trials in real time to see which are enrolling successfully and which are struggling. And unlike using a CTMS alone, PROPEL allows you to drill down into specific metrics with powerful reporting by trial, site, or organization.
Performance Insights
Throughout the clinical trial process, PROPEL captures real-time metrics every step of the way, providing full-cycle, wrap-around performance insights and data analytics. Using customizable reports and dashboards, users gain powerful insights into turnaround timing, enrollment progress, and overall performance.
After a trial is completed, PROPEL enables central operations and leadership teams to dig into past performance to help with future projections and predictions. Report on historical enrollment performance segmented by indication, contract percentage, and other trial characteristics. Use these insights to make future decisions during site selection and share them with CROs and sponsors to help win more trial opportunities.
Experience time and cost savings, improved efficiency, increased productivity, and full performance transparency throughout the entire clinical trial process with PROPEL. Schedule a personalized demo today to learn how your organization can streamline processes and secure more of the right opportunities.
Devana Solutions
Devana Solutions is an innovative cloud-based clinical trial software company that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Devana Solutions bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trials systems.
Let us show you in more detail what Better Data, Better Decisions, Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.