2023 SCRS Global Site Solutions Summit: Forging Stronger Sites
The SCRS Global Site Solutions Summit is almost upon us. By design, the Summit aims to help make sites of all sizes and structures more competitive and successful. This year, topics range from strategies for improving retention rates, to contract and budget help. As a Premier Sponsor of the Summit, Devana Solutions is proud to be part of these annual discussions around expanding site capabilities and resources.
Become a More Competitive Site
Want your site organization to stand out more during feasibility? Be sure to attend Breakout Session IV: How to Stand Out as a Site and Be Selected for Studies on Saturday, October 7 at 4:15pm. 
Join Devana Solutions’ CEO and Co-Founder Barry Lake as he facilitates a panel of successful sites and sponsors as they discuss how to become a partner site of choice. Understand the key elements of the study startup process and get tips and metrics from sponsors and CROs to help improve your site’s visibility. Featured panelists include Jillian Agnew, Senior Clinical Research Nurse, St. Johns Center for Clinical Research, Karen McIntyre, Global Site Alliances, Parexel, Lindsey Morales, Associate Director of Clinical Operations, Gilead, and Karen Pypniowski, Vice President of Feasibility & Site Operations, Circuit Clinical.
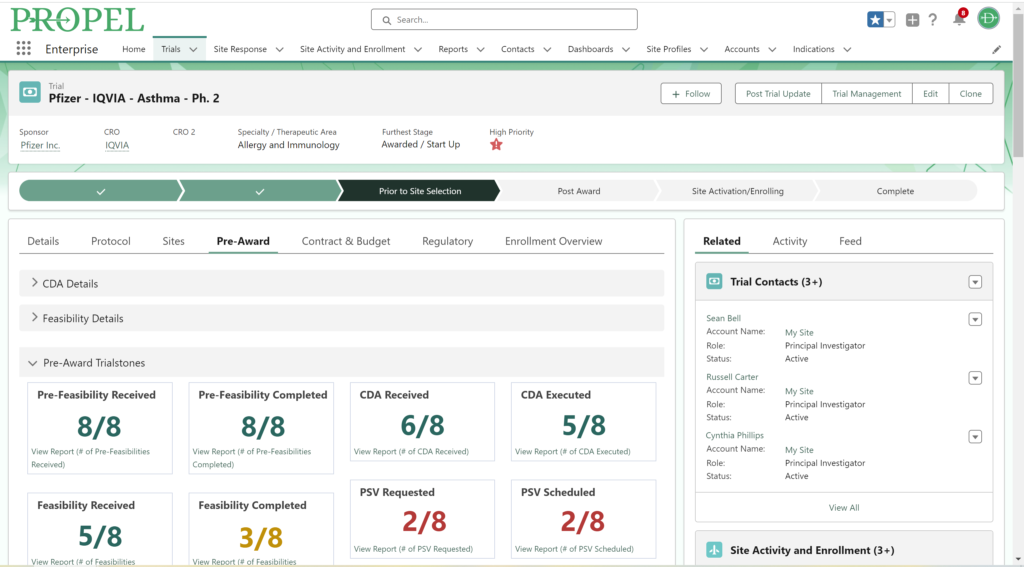
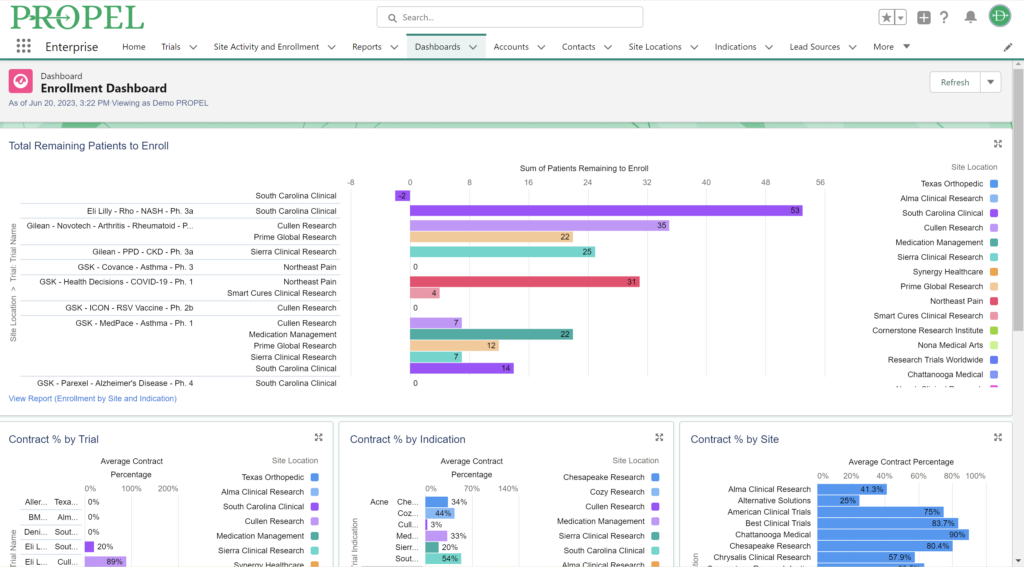
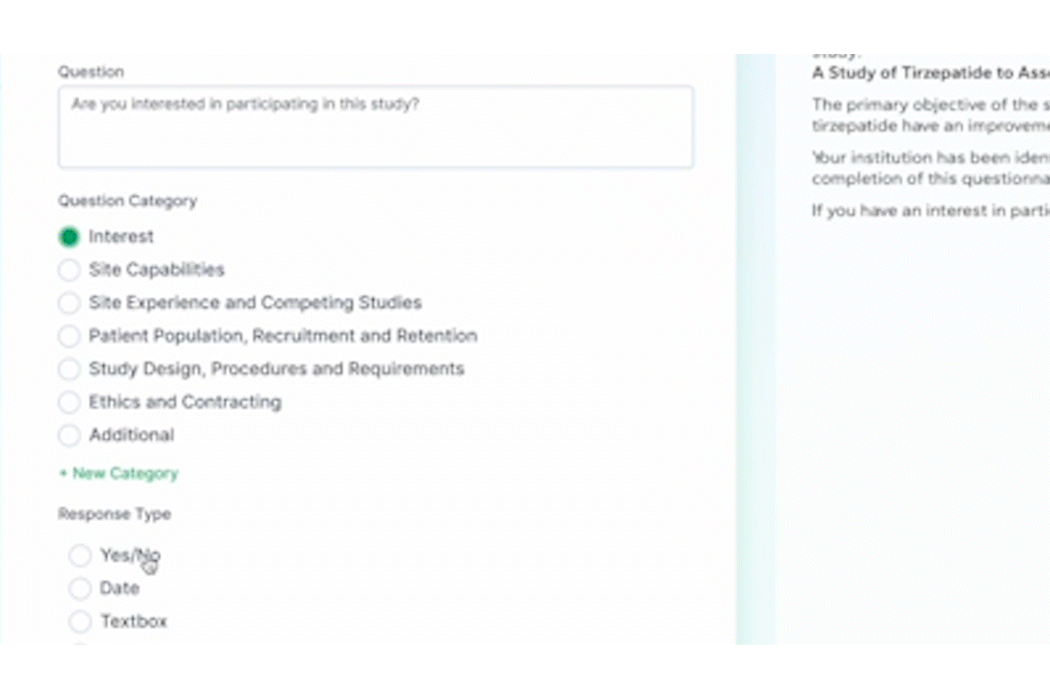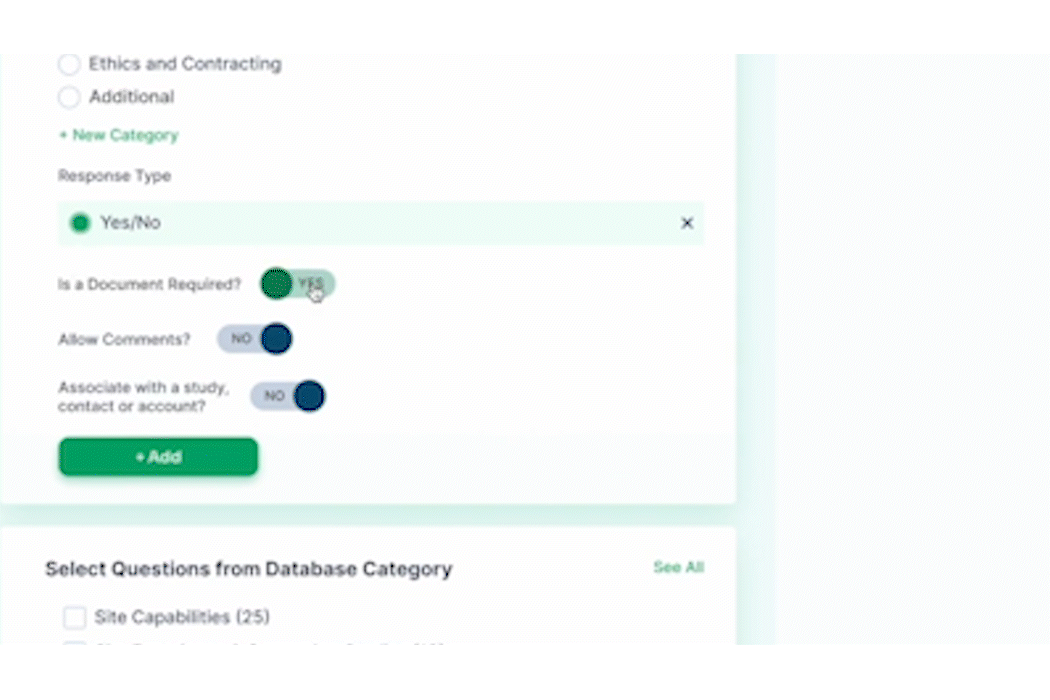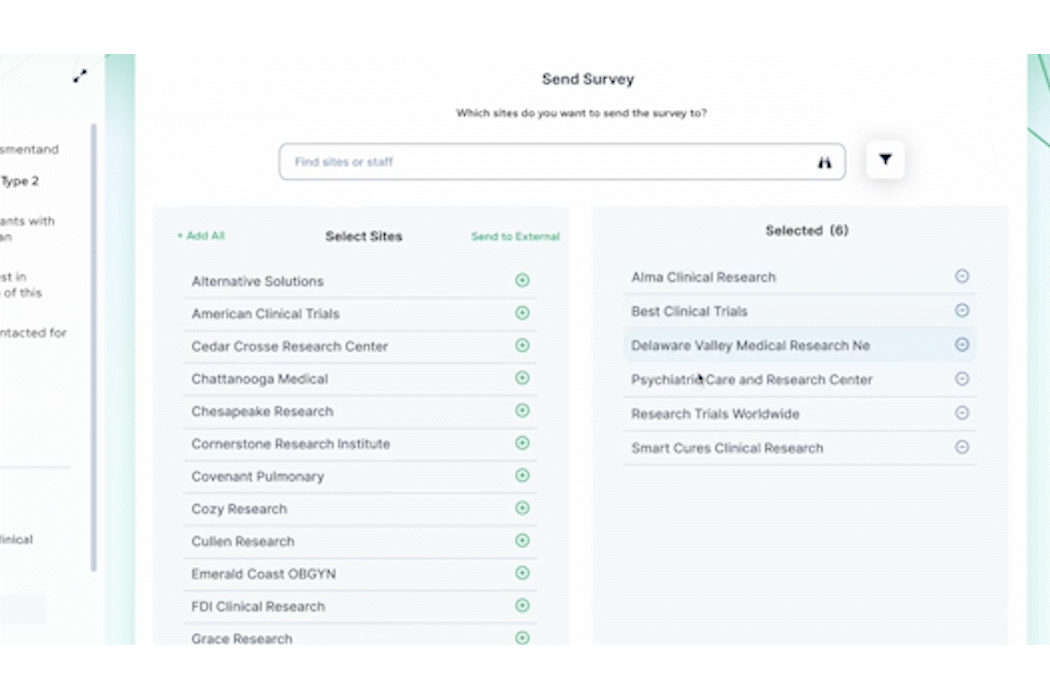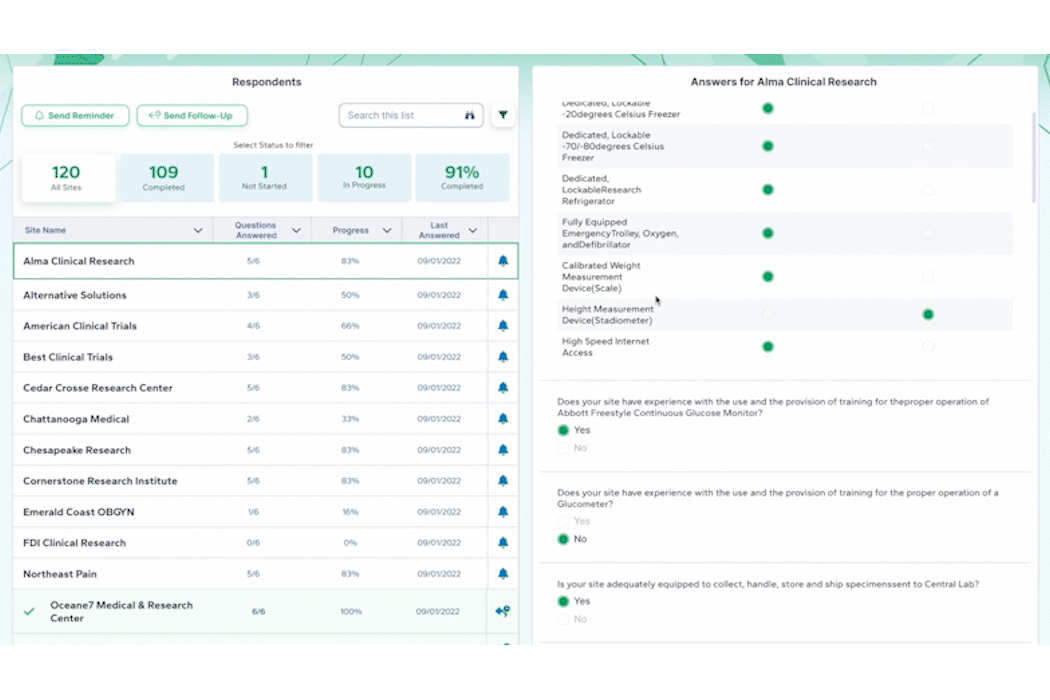
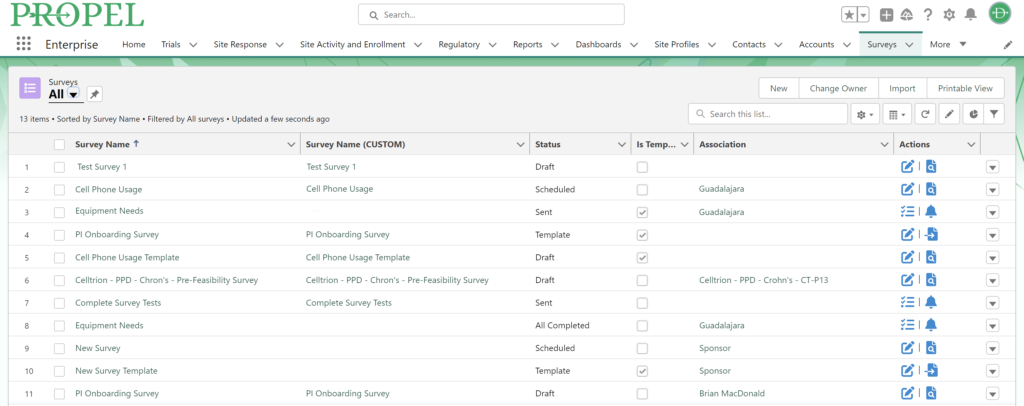
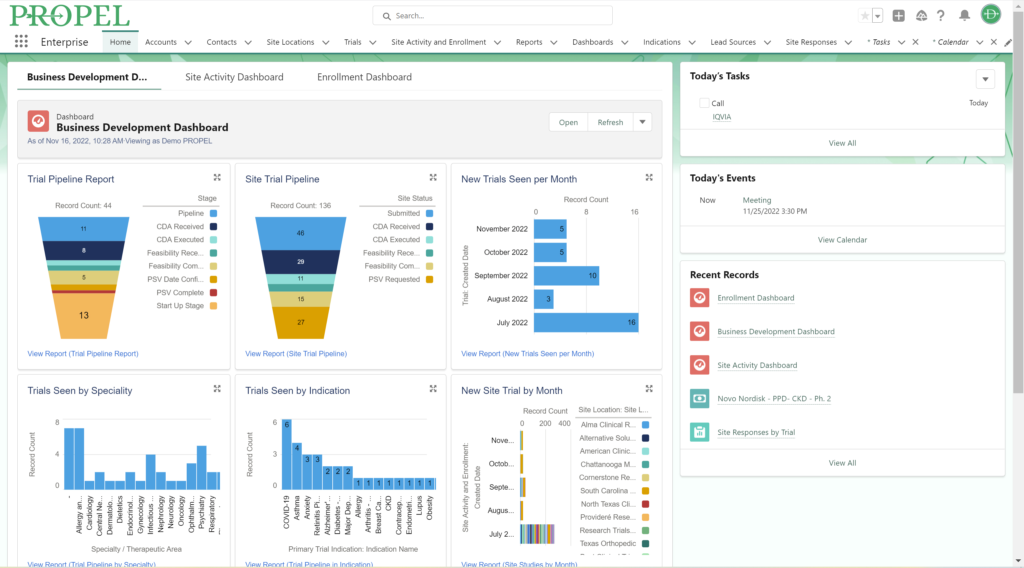
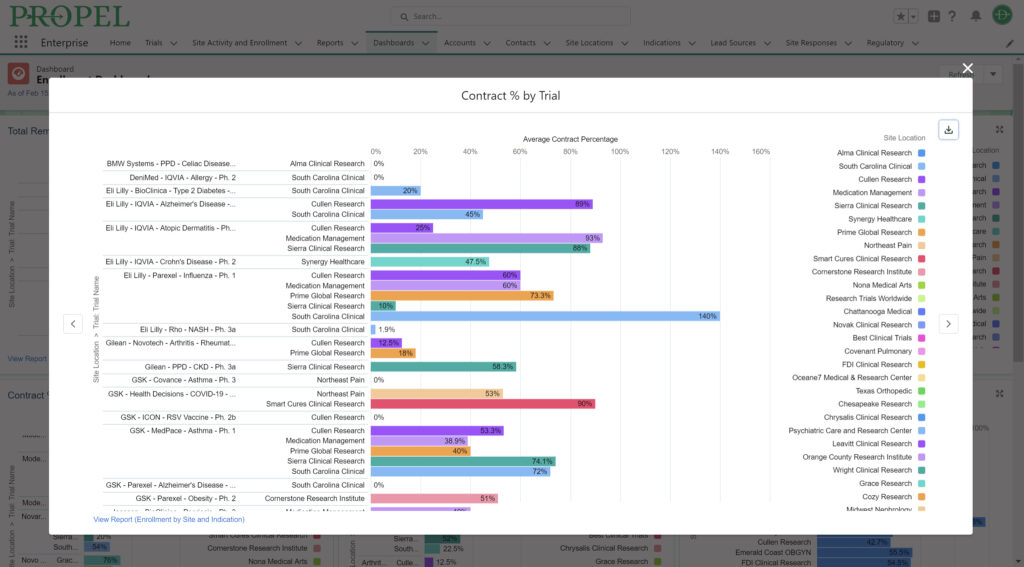
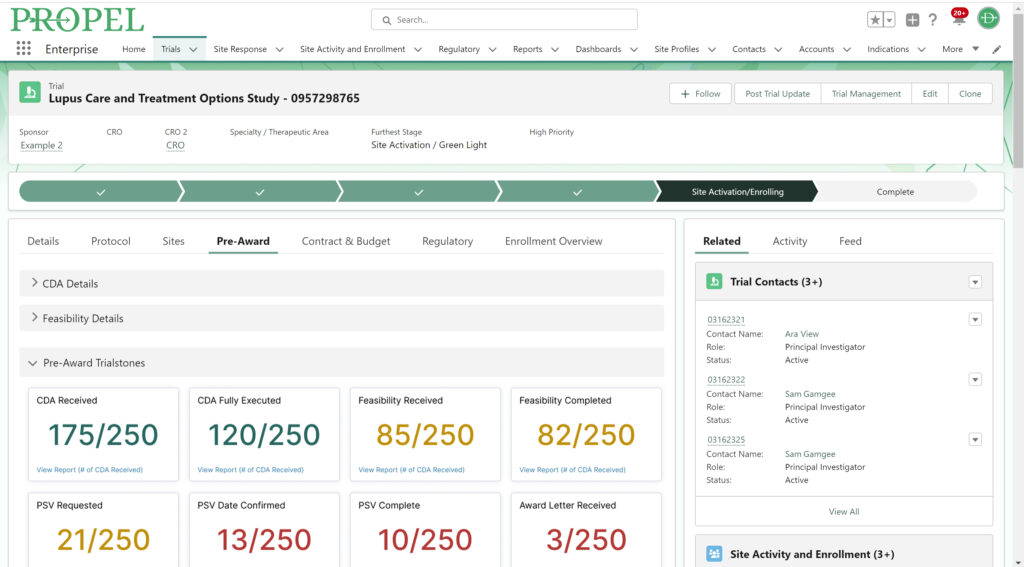
And don’t forget to visit Team Devana at Booth 507 in the Expo Hall for a demo of PROPEL, our comprehensive clinical trial data management software. Let us show you how workflow automation and advanced data analytics can transform your organization’s clinical trial process end-to-end and give you a leading edge on winning trial opportunities.
Devana Solutions
Devana Solutions is an innovative cloud-based clinical trial software company that supports real-time collaboration between central research operations professionals and decentralized clinicians serving patients in diverse communities. Our platform bridges the technology access and data divide by seamlessly and securely connecting decentralized researchers to other mission-critical clinical trial systems.
Let us show you in more detail what Better Data, Better Decisions, and Better Outcomes could do for your clinical trial processes. Book a demo with Devana Solutions today to learn about our cloud-based data analytics clinical trial software for the clinical trials industry that integrates seamlessly with CTMS and other key systems to keep success in the crosshairs.